LOAD MORE
You're viewed 9 of 22 products
Daicel Pharma offers worldwide delivery options for a custom synthesis of Isavuconazole impurity standards, including crucial impurity standards such as (2-(methylamino)pyridin-3-yl)methanol, Isavuconazole Diastereomer-1, Isavuconazole Enantiomer, Isavuconazole Impurity 14, Isavuconazole Impurity 36, Isavuconazole Impurity-6, Isavuconazonium chloride(diasteriomer-1), and many more. These impurity standards play a vital role in evaluating the purity and safety of Isavuconazole, an active pharmaceutical ingredient.
Isavuconazole [CAS: 241479-67-4] is a water-soluble triazole prodrug known for its broad-spectrum antifungal activity. It functions as an inhibitor of ergosterol biosynthesis and targets sterol 14alpha-demethylase, an enzyme involved in the synthesis of ergosterol, an essential component of fungal cell membranes.
Isavuconazole, an approved treatment for invasive aspergillosis and invasive mucormycosis, is a water-soluble triazole prodrug with broad-spectrum antifungal activity. It hydrolyzes plasma esterases to form its active component, BAL4815. BAL4815 inhibits the fungal enzyme cytochrome P450 lanosterol 14-alpha-demethylase (CYP51) responsible for converting lanosterol to ergosterol, a part of the fungal cell membrane. By inhibiting CYP51, BAL4815 reduces the pool of ergosterol, disrupting the synthesis of the fungal cell membrane. Isavuconazole is a clinical alternative to voriconazole for treating invasive aspergillosis. Isavuconazole is available under Cresemba.
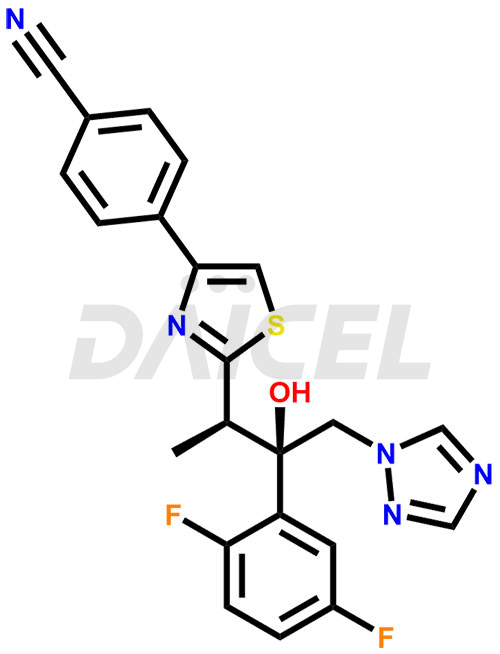
The chemical name of Isavuconazole is 4-[2-[(1R,2R)-2-(2,5-Difluorophenyl)-2-hydroxy-1-methyl-3-(1H-1,2,4-triazol-1-yl)propyl]-4-thiazolyl]benzonitrile (ACI) (2R,3R)-3-[4-(4-Cyanophenyl)thiazol-2-yl]-2-(2,5-difluorophenyl)-1-(1H-1,2,4-triazol-1-yl)butan-2-ol. Its chemical formula is C22H17F2N5OS, and its molecular weight is approximately 437.5 g/mol.
Isavuconazole inhibits the formation of ergosterol, a component of fungal cell membranes. This disruption increases the membrane permeability causing the loss of essential intracellular parts. Ultimately, the fungal cells undergo lysis and die.
As with any pharmaceutical compound, Isavuconazole can potentially contain impurities. Impurities in Isavuconazole can arise from various sources, such as during synthesis1, starting materials, intermediates, or degradation products. The common Isavuconazole impurities include related substances, residual solvents, and degradation products. They may arise from incomplete reactions, impure starting materials, or storage conditions. It is necessary to control and monitor these impurities to ensure the safety and efficacy of the medication. Pharmaceutical regulatory authorities, such as the United States Food and Drug Administration (FDA), have established guidelines and limits in pharmaceuticals, including Isavuconazole. Manufacturers comply with these regulations and conduct thorough testing to identify and quantify impurities within acceptable limits.
Daicel Pharma strictly adheres to cGMP standards and operates an analytical facility for preparing Isavuconazole impurity standards. We provide a range of Isavuconazole impurity standards, such as (2-(methylamino)pyridin-3-yl)methanol, Isavuconazole Diastereomer-1, Isavuconazole Enantiomer, Isavuconazole Impurity 14, Isavuconazole Impurity 36, Isavuconazole Impurity-6, Isavuconazonium chloride(diasteriomer-1), and many more. Our impurity standards have a detailed Certificate of Analysis (CoA) and a comprehensive characterization report. The CoA encompasses data obtained through techniques, 1H NMR, 13C NMR, IR, MASS, and HPLC purity analysis2. Additional data, such as 13C-DEPT, can be provided upon request. We can synthesize unknown Isavuconazole impurity standards, degradation products, and labeled compounds, to evaluate the efficacy of generic Isavuconazole. Additionally, we offer a highly pure Isotope of Oxazine Imp of Isavuconazole, deuterium-labeled Isavuconazole standards for bioanalytical research and BA/BE studies. Each delivery has a comprehensive characterization report.
Impurities in Isavuconazole are identified using analytical techniques such as chromatography, spectroscopy, and mass spectrometry. Comparison with reference standards or known impurities aids in identification.
Impurities in Isavuconazole are tested and monitored throughout the synthesis. Regular testing ensures compliance with regulatory guidelines and maintains the quality of the medication.
Quantification of impurities in Isavuconazole uses validated analytical methods. Calibration curves are generated using known reference standards to determine their concentration.
The recommendation is to store Isavuconazole impurities at a controlled room temperature, within 2-8 °C.
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.