LOAD MORE
You're viewed 9 of 22 products
Daicel Pharma synthesizes Dexmedetomidine impurities of exceptional quality, such as Bromomedetomide impurity, Dexmedetomidine N-Methyl ester Impurity, Dexmedetomidine Potential impurity 5, Dibromomedetomide impurity, etc. These impurities are crucial to assess the purity, reliability, and safety of Dexmedetomidine, an active pharmaceutical ingredient. Besides, Daicel Pharma provides custom synthesis of Dexmedetomidine impurities to meet clients’ demands for delivery worldwide.
Dexmedetomidine [CAS: 113775-47-6], an imidazole derivative, is the S-enantiomer of medetomidine. It is an alpha-adrenergic agonist, providing analgesic, anxiolytic, and sedative effects. It is closely related to medetomidine, the racemic form of this compound. Dexmedetomidine treats agonistic action on adrenergic alpha-2 receptors, offering non-narcotic analgesic properties.
Dexmedetomidine, available under various brand names such as Igalmi, and Precedex, has received US FDA approval for specific uses. They involve sedation for intubated and mechanically ventilated patients in the ICU and peri-procedural sedation for non-intubated patients. Dexmedetomidine has even been utilized in peripheral nerve blocks to extend the duration of analgesia.
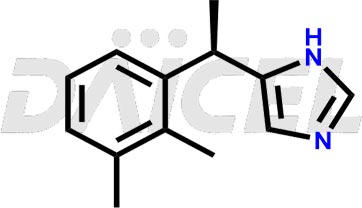
The chemical name of Dexmedetomidine is 5-[(1S)-1-(2,3-Dimethylphenyl)ethyl]-1H-imidazole. Its chemical formula is C13H16N2, and its molecular weight is approximately 200.28 g/mol.
Dexmedetomidine activates presynaptic alpha-2 adrenergic receptors that treat agitation associated with schizophrenia or bipolar I or II disorder.
During the synthesis1 of Dexmedetomidine, impurities may form as byproducts. They can arise from various steps in the manufacturing process, such as starting materials, reagents, or reaction intermediates. It helps identify and characterize these impurities to ensure the safety and efficacy of the final product. Analytical techniques like high-performance liquid chromatography (HPLC) and mass spectrometry (MS) help in impurity profiling and quantification. By understanding the impurities present in Dexmedetomidine and their levels, manufacturers can optimize the synthetic process and implement appropriate purification strategies to meet regulatory requirements and ensure the highest quality of the drug.
Daicel Pharma offers a Certificate of Analysis (CoA) for Dexmedetomidine impurity standards, such as Bromomedetomide impurity, Dexmedetomidine N-Methyl ester Impurity, Dexmedetomidine Potential impurity 5, Dibromomedetomide impurity, etc., generated from an analytical facility compliant with cGMP standards. The CoA includes a comprehensive characterization report comprising data2 from techniques like 1H NMR, 13C NMR, IR, MASS, and HPLC purity3. Furthermore, on request, we give additional data like 13C-DEPT and CHN. Daicel Pharma can synthesize unknown Dexmedetomidine impurities or degradation products. A complete characterization report accompanies every delivery.
Dexmedetomidine impurities help ensure the drug's quality, safety, and efficacy. Controlling impurities help maintain the desired pharmacological properties, reduces the risk of adverse effects, and ensures compliance with regulatory standards.
Impurities in Dexmedetomidine are analyzed using various analytical techniques such as chromatography (HPLC, LC), spectroscopy (UV, IR), mass spectrometry (MS), and other advanced methods. These techniques help in the identification, quantification, and characterization of impurities.
Methanol and Water are the solvents used in analyzing many impurities in Dexmedetomidine.
Dexmedetomidine impurities are stored at a controlled room temperature between 2-8 °C or as indicated on the Certificate of Analysis (CoA).
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.