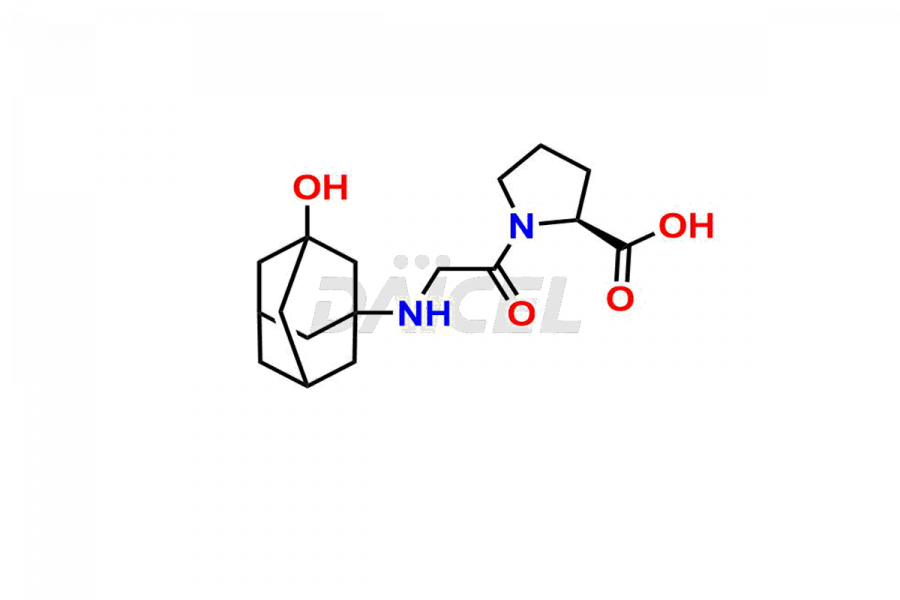
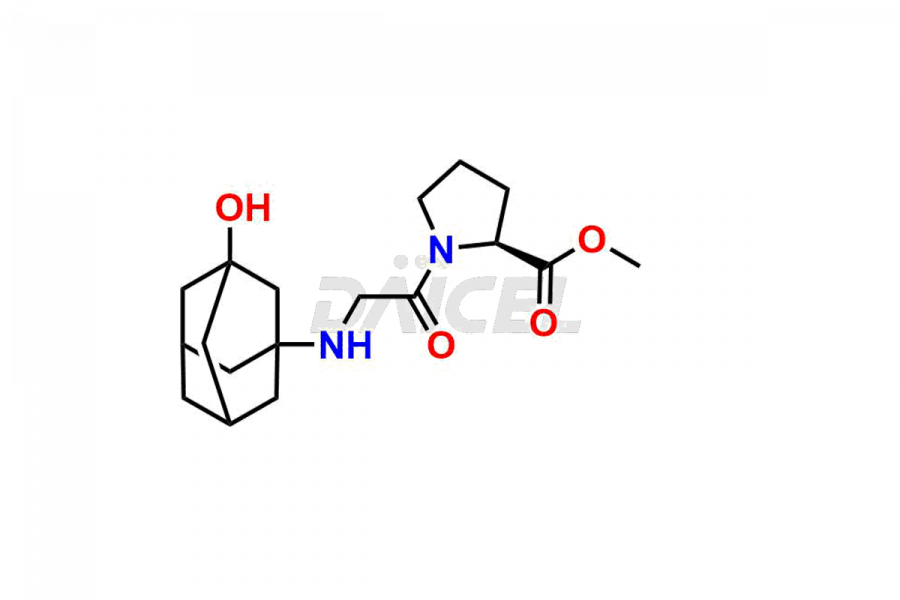Vildagliptin
References
- Villhauer, Edwin Bernard, N-Substituted 2-Cyanopyrrolidines, Novartis A.-G., Switzerland, EP1137635B1, October 19, 2005
- Barden, Amanda Thomas; Salamon, Barbara; Schapoval, Elfrides Eva Sherman; Steppe, Martin, Stability-Indicating RP-LC Method for the Determination of Vildagliptin and Mass Spectrometry Detection for a Main Degradation Product, Journal of Chromatographic Science Volume: 50, Issue: 5, Pages: 426-432, 2012
Frequently Asked Questions
Why is it essential to control impurities in Vildagliptin?
Controlling impurities in Vildagliptin ensures the drug’s quality, safety, and efficacy. They can affect Vildagliptin stability, pharmacological activity, and toxicity and interfere with the analytical methods used for its detection and quantification.
What are the analytical methods used to detect Vildagliptin impurities?
High-performance liquid chromatography-mass spectrometry helps accurately measure the levels of the impurities in Vildagliptin.
What is the acceptable limit for Vildagliptin impurities?
Regulatory agencies like the US FDA, EMA, and ICH establish acceptable limits for impurities in Vildagliptin. The limits may vary depending on the impurities ensuring that Vildagliptin meets stringent standards for its quality and suitability for patient use.
How are Vildagliptin impurities controlled during the synthesis of the drug?
Impurities in Vildagliptin are controlled during the synthetic process by implementing good manufacturing practices (GMP) and using appropriate analytical methods for impurity identification and quantification.
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.