LOAD MORE
You're viewed 9 of 20 products
Daicel Pharma is a trusted provider of Vildagliptin impurity standards, including (2R)-1-(Chloroacetyl)-2-pyrrolidinecarbonitrile, 1-(2-chloro-2-(((1R,3S,5R)-3-hydroxyadamantan-1-yl)amino)acetyl) pyrrolidine-2-carbonitrile,3-Amino-1-adamantanol, VDN-Dihydroxy Impurity, 1-(((1R,3R,5S)-3,5-dihydroxyadamantan-1-yl)glycyl) pyrrolidine-2-carbonitrile, Vildagliptin Mono Keto Impurity, Vildagliptin related compound A, and many more. These impurities are crucial in meticulously evaluating the quality, stability, and safety of the active pharmaceutical ingredient, Vildagliptin. Furthermore, Daicel Pharma customizes Vildagliptin impurities, guaranteeing to meet client specifications. With global shipping capabilities, these impurities can be conveniently delivered to customers worldwide, offering unparalleled convenience.
Vildagliptin [CAS: 274901-16-5] enhances glycemic control in adults with type 2 diabetes mellitus as a supplementary treatment to diet and exercise. It is used alone in patients who cannot take metformin due to contraindications or intolerance.
Vildagliptin is a medication used to treat type 2 diabetes mellitus in adults. It helps to improve glycemic control by maintaining stable blood sugar levels. Vildagliptin is for adults who require additional glycemic control beyond what insulin alone can achieve. Vildagliptin is available under Jalra, Xiliarx, etc.
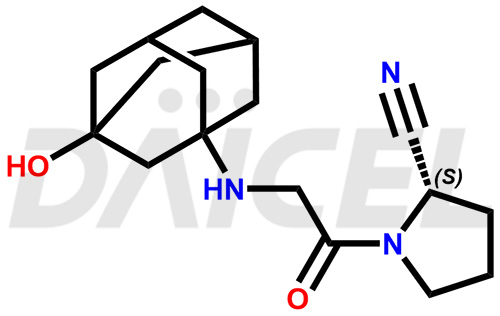
The chemical name of Vildagliptin is (2S)-1-[2-[(3-Hydroxytricyclo[3.3.1.13,7]dec-1-yl)amino]acetyl]-2-pyrrolidinecarbonitrile. Its chemical formula is C17H25N3O2, and its molecular weight is approximately 303.4 g/mol.
Vildagliptin selectively inhibits the enzyme dipeptidyl peptidase-4 (DPP-4) that inactivates the release of Glucagon-like peptide-1 (GLP-1) and incretin hormones from intestinal cells.
Vildagliptin impurities can arise during synthesis1 due to the storage or use of specific raw materials and intermediates in manufacturing. These impurities encompass related compounds, degradation products, and process impurities. Stringent quality control measures and analytical methods are crucial to ensure the purity and safety of Vildagliptin for patient use.
Daicel Pharma provides a comprehensive Certificate of Analysis (CoA) for Vildagliptin impurity standards, such as (2R)-1-(Chloroacetyl)-2-pyrrolidinecarbonitrile, 1-(2-chloro-2-(((1R,3S,5R)-3-hydroxyadamantan-1-yl)amino)acetyl) pyrrolidine-2-carbonitrile,3-Amino-1-adamantanol, VDN-Dihydroxy Impurity, 1-(((1r,3R,5S)-3,5-dihydroxyadamantan-1-yl)glycyl) pyrrolidine-2-carbonitrile, Vildagliptin Mono Keto Impurity, Vildagliptin related compound A, and many more. The CoA includes detailed characterization data such as 1H NMR, 13C NMR, IR, MASS, and HPLC purity2. Additionally, upon delivery, we give 13C-DEPT. Daicel possesses the technology and expertise to synthesize any unknown Vildagliptin impurity or degradation product.
Controlling impurities in Vildagliptin ensures the drug’s quality, safety, and efficacy. They can affect Vildagliptin stability, pharmacological activity, and toxicity and interfere with the analytical methods used for its detection and quantification.
High-performance liquid chromatography-mass spectrometry helps accurately measure the levels of the impurities in Vildagliptin.
Regulatory agencies like the US FDA, EMA, and ICH establish acceptable limits for impurities in Vildagliptin. The limits may vary depending on the impurities ensuring that Vildagliptin meets stringent standards for its quality and suitability for patient use.
Impurities in Vildagliptin are controlled during the synthetic process by implementing good manufacturing practices (GMP) and using appropriate analytical methods for impurity identification and quantification.
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.