LOAD MORE
You're viewed 9 of 12 products
Daicel Pharma is a reliable partner for synthesizing high-quality Varenicline impurities, specifically 1-((1R,5S)-8-amino-6-nitro-1,2,4,5-tetrahydro-3H-1,5-methanobenzo[d]azepin-3-yl)-2,2,2-trifluoroethan-1-one, Methyl Varenicline Impurity, N-Nitroso Varenicline, Varenicline Impurity II, Trifluoroacetyl Varenicline Hydroxymethyl Impurity, Varenicline tartrate adduct and more. These impurities help assess the quality, stability, and safety of the active pharmaceutical ingredient, Varenicline. Daicel Pharma also offers custom synthesis of Varenicline impurities, which can be shipped worldwide.
Varenicline [CAS: 249296-44-4] is a medication that acts as a partial agonist of the nicotinic acetylcholine receptor, alpha4beta2. It helps in giving up smoking.
Varenicline is an organic compound similar to cytisine that acts as a partial agonist for nicotinic cholinergic receptors. It is available in its tartrate salt form. Varenicline helps as a smoking cessation aid, with patients undergoing education and counseling in conjunction with its use. It is considered the first line of treatment for smoking cessation due to its ability to prevent short-term and long-term relapse. Varenicline appears more effective than bupropion and equally effective as nicotine replacement therapy. Additionally, Varenicline has a lower risk of withdrawal symptoms due to its partial agonist properties. Some of the brand names for Varenicline include Chantix and Tyrvaya.
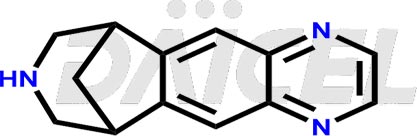
The chemical name of Varenicline is 7,8,9,10-Tetrahydro-6,10-methano-6H-pyrazino[2,3-h][3]benzazepine. Its chemical formula is C13H13N3, and its molecular weight is approximately 211.26 g/mol.
Varenicline binds selectively and with affinity at α4β2 neuronal nicotinic acetylcholine receptors. It ends smoking in patients and prevents nicotine binding at α4β2 receptors.
During the synthesis1 and storage of Varenicline, impurities may form that can affect the drug’s safety and efficacy. Some impurities include related substances, starting materials, and degradation products. The impurity control is through proper process development, purification, and storage conditions.
Daicel Pharma issues a Certificate of Analysis (CoA) for Varenicline impurity standards, including 1-((1R,5S)-8-amino-6-nitro-1,2,4,5-tetrahydro-3H-1,5-methanobenzo[d]azepin-3-yl)-2,2,2-trifluoroethan-1-one, Methyl Varenicline Impurity, N-Nitroso Varenicline, Varenicline Impurity II, Trifluoroacetyl Varenicline Hydroxymethyl Impurity, Varenicline tartrate adduct and more. The CoA is from an analytical facility that complies with current Good Manufacturing Practices (cGMP) and includes comprehensive characterization data, such as 1H NMR, 13C NMR, IR, MASS, and HPLC purity2. We give additional characterization data such as 13C-DEPT and CHN on request. Daicel Pharma can prepare unknown Varenicline impurities or degradation products. A complete characterization report accompanies each delivery.
HPLC separates impurities based on their chemical and physical properties, such as molecular weight, polarity, and charge. The separated impurities are then detected using a UV detector or a mass spectrometer.
Mass spectrometry is a sensitive technique that can detect and quantify impurities in Varenicline at low levels. It provides information on the molecular weight and structure of the impurities that helps in their identification and characterization.
Methanol or Acetonitrile is a solvent used in analyzing many impurities in Varenicline.
Varenicline impurities are stored at a controlled room temperature between 2-8 ⁰C or as indicated on the Certificate of Analysis (CoA).
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.