LOAD MORE
You're viewed 9 of 17 products
Daicel Pharma is a trusted provider of quality Valsartan impurity standards, including 4′-(azidomethyl)-[1,1′-biphenyl]-2-carboxamide,4′-(bromomethyl)-[1,1′-biphenyl]-2-carbonitrile, R-Valsartan, Valsartan Impurity F, Valsartan Related compound B, and many more. These impurities are crucial in meticulously evaluating the quality, stability, and safety of the active pharmaceutical ingredient, Valsartan. Furthermore, Daicel Pharma customizes Valsartan impurities, guaranteeing that it meets client specifications. With global shipping capabilities, these impurities can be conveniently delivered to customers worldwide, offering unparalleled convenience.
Valsartan [CAS: 137862-53-4] treats hypertension and lowers the chances of cardiovascular incidents such as strokes and heart attacks. It treats heart failure in patients intolerant of angiotensin-converting enzyme (ACE) inhibitors.
Valsartan is an angiotensin II receptor blocker used alone or combined with other antihypertensive agents to treat hypertension and reduce cardiovascular mortality after myocardial infarction. It lowers blood pressure and reduces the risk of cardiovascular events such as strokes and heart attacks. Valsartan is available under the brand names Diovan and Prexxartan.
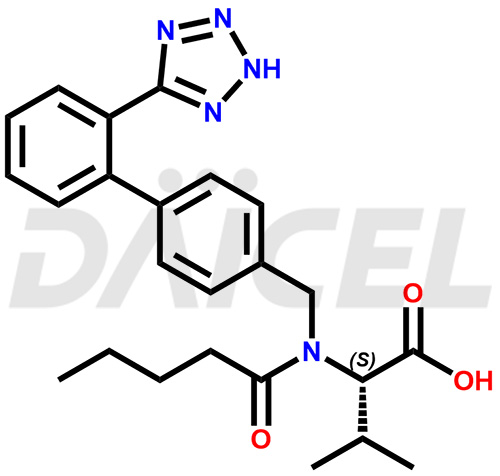
The chemical name of Valsartan is N-(1-Oxopentyl)-N-[[2′-(2H-tetrazol-5-yl)[1,1′-biphenyl]-4-yl]methyl]-L-valine. Its chemical formula is C24H29N5O3, and its molecular weight is approximately 435.5 g/mol.
Valsartan selectively blocks the binding of angiotensin II to the AT1 receptor in many tissues. It prevents the vasoconstrictor and aldosterone-secreting effects of angiotensin II.
Valsartan impurities can arise during synthesis1 due to the storage or use of specific raw materials and intermediates in manufacturing. These impurities encompass related compounds, degradation products, and process impurities. Stringent quality control measures and analytical methods are crucial to ensure the purity and safety of Valsartan for patient use.
Daicel Pharma provides a comprehensive Certificate of Analysis (CoA) for Valsartan impurity standards, such as 4′-(azidomethyl)-[1,1′-biphenyl]-2-carboxamide,4′-(bromomethyl)-[1,1′-biphenyl]-2-carbonitrile, R-Valsartan, Valsartan Impurity F, Valsartan Related compound B, and many more. The CoA includes detailed characterization data such as 1H NMR, 13C NMR, IR, MASS, and HPLC purity2. Additionally, upon delivery, we give 13C-DEPT. Daicel possesses the technology and expertise to synthesize any unknown Valsartan impurity or degradation product. We also offer labeled compounds to quantify the efficacy of generic Valsartan. For bioanalytical research and BA/BE studies, Daicel supplies highly pure S-Valsartan-D9, deuterium-labeled compounds of Valsartan.
High-performance liquid chromatography (HPLC) and LCMS/ MS are employed to separate and analyze the impurities of Valsartan.
Impurities found in Valsartan can be classified into several categories, which include process-related contaminants, degradation products, residual solvents, and genotoxic impurities.
Valsartan impurities can change over time, influenced by storage conditions, exposure to light, and elevated temperatures.
The recommendation is to store Valsartan impurities at room temperature, within 2-8 °C.
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.