LOAD MORE
You're viewed 9 of 20 products
Daicel Pharma is a reliable source for synthesizing high-quality Ticagrelor impurities like (1S,2S,3R,5S)-3-((5-amino-2-(propylthio)pyrimidin-4-yl)amino)-5-(2-hydroxyethoxy)cyclopentane-1,2-diol,2-(2,3-difluorophenyl)cyclopropanamine hydrochloride, 2-(3,5-difluorophenyl)cyclopropanamine hydrochloride, O-acetyl Ticagrelor, Ticagrelor acetonide, Ticagrelor amine impurity AR-C133913XX and many more. These impurities are essential in accurately analyzing the Ticagrelor quality, stability, and biological safety. Additionally, Daicel Pharma offers a customized synthesis of Ticagrelor impurities for global delivery to meet the specific needs of our customers.
Ticagrelor [CAS: 274693-27-5] is a P2Y12 receptor antagonist. It lowers the likelihood of myocardial infarction and ischemic stroke.
Ticagrelor lowers the risk of myocardial infarction and ischemic stroke by inhibiting thrombus formation. This medication reduces the risk of cardiovascular death, myocardial infarction, and stroke in individuals with acute coronary syndrome or a history of myocardial infarction.
Ticagrelor is available under Brilinta, which contains the active ingredient, Ticagrelor.
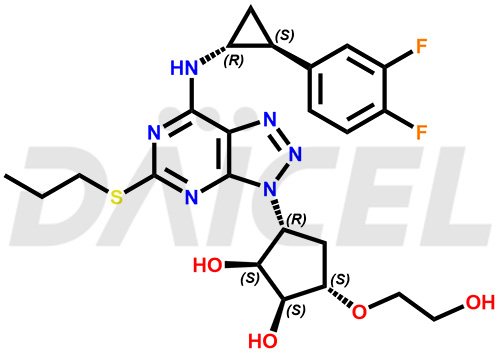
The chemical name of Ticagrelor is (1S,2S,3R,5S)-3-[7-[[(1R,2S)-2-(3,4-Difluorophenyl)cyclopropyl]amino]-5-(propylthio)-3H-1,2,3-triazolo[4,5-d]pyrimidin-3-yl]-5-(2-hydroxyethoxy)-1,2-cyclopentanediol. Its chemical formula is C23H28F2N6O4S, and its molecular weight is approximately 522.6 g/mol.
Ticagrelor prevents signal transduction and platelet activation by reversibly interacting with the platelet P2Y12 ADP receptor.
During Ticagrelor manufacturing1, impurity formation is possible, compromising its effectiveness. They can arise from various sources, including the raw materials, intermediates, and chemicals utilized to synthesize Ticagrelor. To ensure the drug’s optimal efficacy and safety, closely managing and monitoring these impurities is paramount.
Daicel offers a wholesome and integrated Certificate of Analysis (CoA) for Ticagrelor impurity standards, encompassing(1S,2S,3R,5S)-3-((5-amino-2-(propylthio)pyrimidin-4-yl)amino)-5-(2-hydroxyethoxy)cyclopentane-1,2-diol, 2-(2,3-difluorophenyl)cyclopropanamine hydrochloride, 2-(3,5-difluorophenyl)cyclopropanamine hydrochloride, O-acetyl Ticagrelor, Ticagrelor acetonide, Ticagrelor amine impurity AR-C133913XX and many more. The Certificate of Analysis (CoA) offers detailed characterization data, including 1H NMR, 13C NMR, IR, MASS, and HPLC purity2, ensuring comprehensive information about the product. Furthermore, Daicel Pharma possesses advanced technological capabilities and expertise to synthesize any unknown impurities or degradation products of Ticagrelor.
Analytical methods, such as high-performance liquid chromatography (HPLC), are commonly used to detect and quantify impurities in Ticagrelor batches.
The specifications for Ticagrelor impurities are determined through meticulous research, analysis, and adherence to regulatory guidelines. Essential factors, including toxicological studies, clinical data, and regulatory considerations, are thoroughly evaluated to establish acceptable limits for impurity levels.
A chiral column separation technique helps identify the chiral isomer and employs a self-contrast approach without needing a correction factor to quantify impurities. Experimental results demonstrate that the chosen chromatographic conditions effectively separate the main component and its chiral isomer, allowing for better control over the mass of the Ticagrelor crude drug.
Ticagrelor impurities are stored at a controlled room temperature, between 2-8 °C or as indicated on the Certificate of Analysis (CoA).
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.