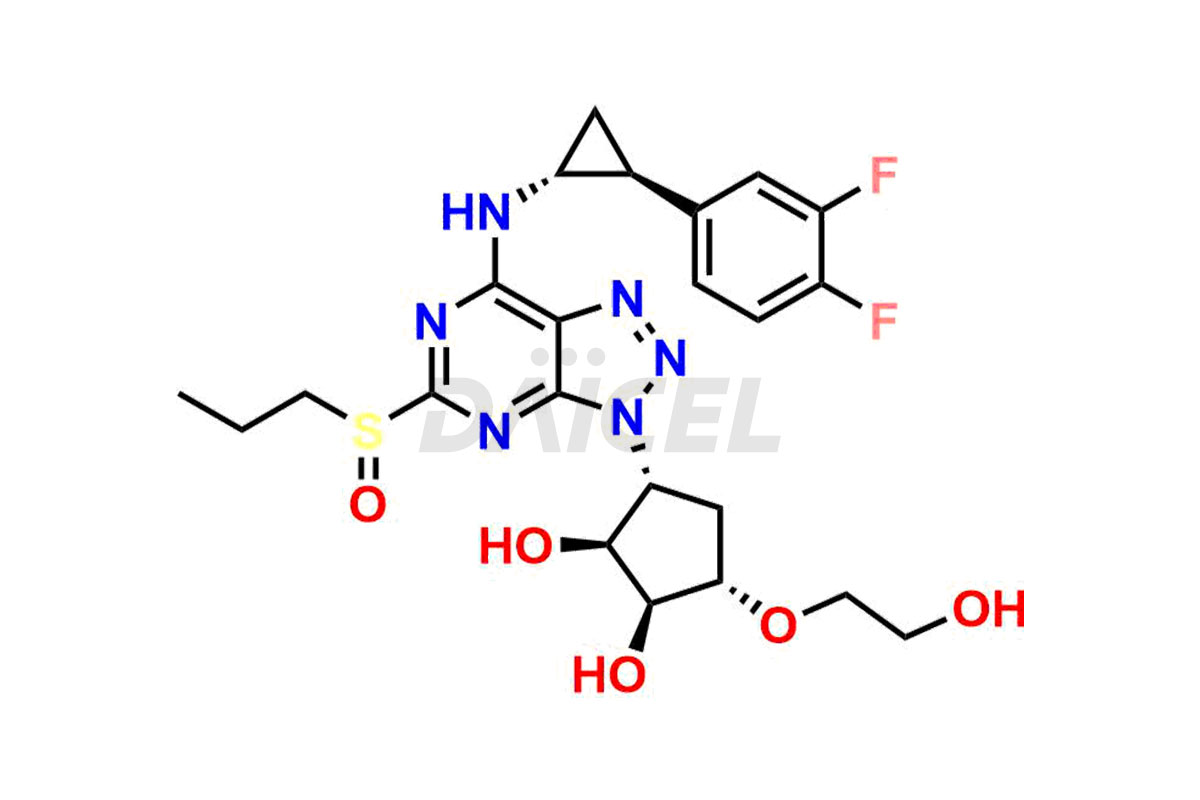
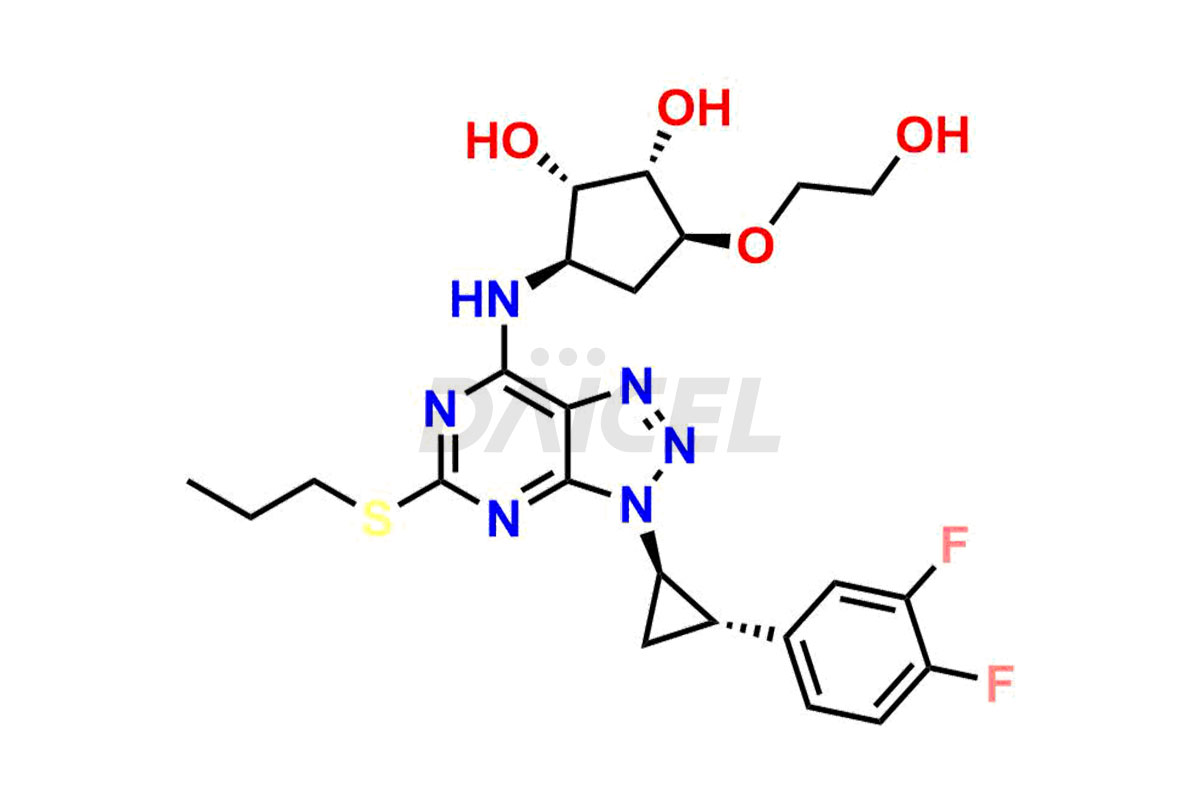
Ticagrelor
References
- Guile, Simon; Hardern, David; Ingall, Anthony; Springthorpe, Brian; Willis, Paul, Novel Triazolo(4,5-D)Pyrimidine Compounds, AstraZeneca UK Limited, United Kingdom, EP1135391B1, March 17, 2004
- Sillen, Henrik; Cook, Melanie; Davis, Patty, Determination of ticagrelor and 2 metabolites in plasma samples by liquid chromatography and mass spectrometry, Journal of Chromatography B: Analytical Technologies in the Biomedical and Life Sciences, Volume: 878, Issue: 25, Pages: 2299-2306, 2010
Frequently Asked Questions
How are Ticagrelor impurities detected and quantified?
Analytical methods, such as high-performance liquid chromatography (HPLC), are commonly used to detect and quantify impurities in Ticagrelor batches.
How are specifications determined for Ticagrelor impurities?
The specifications for Ticagrelor impurities are determined through meticulous research, analysis, and adherence to regulatory guidelines. Essential factors, including toxicological studies, clinical data, and regulatory considerations, are thoroughly evaluated to establish acceptable limits for impurity levels.
How do chromatographic methods help in the chiral separation of Ticagrelor Impurities?
A chiral column separation technique helps identify the chiral isomer and employs a self-contrast approach without needing a correction factor to quantify impurities. Experimental results demonstrate that the chosen chromatographic conditions effectively separate the main component and its chiral isomer, allowing for better control over the mass of the Ticagrelor crude drug.
What are the temperature conditions required to store Ticagrelor impurities?
Ticagrelor impurities are stored at a controlled room temperature, between 2-8 °C or as indicated on the Certificate of Analysis (CoA).
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.