LOAD MORE
You're viewed 9 of 13 products
Daicel Pharma is a reliable source for synthesizing high-quality Sorafenib impurities, N-Desmethyl Sorafenib, Sorafenib dimethyl ester dimer impurity, Sorafenib Impurity 6, Sorafenib impurity 8, Sorafenib N-Oxide, Sorafenib Impurity-B, Sorafenib Impurity-D, Sorafenib Impurity-E, etc. These impurities are essential in analyzing the active pharmaceutical ingredient Sorafenib’s quality, stability, and biological safety. Additionally, Daicel Pharma specializes in the custom synthesis of Sorafenib impurities, catering to specific client requirements. These high-quality impurities can be shipped globally, offering convenience and flexibility to customers worldwide.
Sorafenib [CAS: 284461-73-0] treats unresectable hepatocellular carcinoma and advanced renal cell carcinoma in patients who have not responded to previous interferon-alpha or interleukin-2-based therapy or are deemed unsuitable for such treatment.
Sorafenib is a versatile medication with multiple indications. It primarily treats hepatocellular carcinoma, a form of liver cancer. Additionally, Sorafenib is for patients with advanced renal cell carcinoma who have not responded to prior interferon-alpha or interleukin-2-based therapy or are deemed unsuitable for such treatment. Furthermore, it is approved for treats progressive, locally advanced, or metastatic, differentiated thyroid carcinoma that does not respond to radioactive iodine therapy. Sorafenib’s wide range of applications makes it a valuable option for patients with different types of cancer.
Sorafenib is available under Nexavar, which contains the active ingredient, Sorafenib.
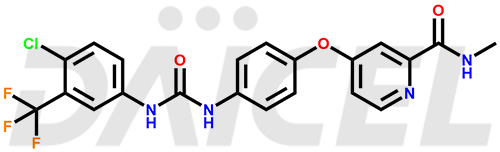
The chemical name of Sorafenib is 4-[4-[[[[4-Chloro-3-(trifluoromethyl)phenyl]amino]carbonyl]amino]phenoxy]-N-methyl-2-pyridinecarboxamide. Its chemical formula is C21H16ClF3N4O3, and its molecular weight is approximately 464.8 g/mol.
Sorafenib inhibits tumor growth of murine renal cell carcinoma.
During Sorafenib’s manufacturing1, impurity formation is possible, compromising its effectiveness. They can arise from various sources, including the raw materials, intermediates, and chemicals utilized to synthesize Sorafenib. Closely managing and monitoring these impurities is paramount to ensure the drug’s optimal efficacy and safety.
Daicel offers a Certificate of Analysis (CoA) for Sorafenib impurity standards, encompassing N-Desmethyl Sorafenib, Sorafenib dimethyl ester dimer impurity, Sorafenib Impurity 6, Sorafenib impurity 8, Sorafenib N-Oxide, Sorafenib Impurity-B, Sorafenib Impurity-D, Sorafenib Impurity-E, etc. The CoA provides detailed characterization data, including 1H NMR, 13C NMR, IR, MASS, and HPLC purity2. Additionally, we give a detailed 13C-DEPT on delivery. With advanced technology and expertise, Daicel can synthesize any unknown Sorafenib impurity or degradation product. We also supply labeled compounds, facilitating the quantification of generic Sorafenib’s efficacy. For bioanalytical research and BA/BE studies, we also offer deuterium-labeled Sorafenib standard Sorafenib D3.
Manufacturers can adopt several strategies to maintain control over impurity levels in Sorafenib. They include using high-quality starting materials, optimizing synthesis and purification processes, implementing comprehensive quality control tests, and continuously monitoring impurity levels at different stages of the manufacturing process.
Inorganic impurities in Sorafenib can be due to the raw materials used during manufacturing, which may include substances such as heavy metals or other contaminants—underscoring the importance of vigilant monitoring and stringent control measures to uphold Sorafenib's quality and safety standards as a pharmaceutical product.
Depending on the type and level of impurities, they can impact the drug's pharmacological activity and stability and pose potential risks to patient health.
Sorafenib Impurities are stored at a controlled room temperature between 2-8 °C or as indicated on the Certificate of Analysis (CoA).
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.