LOAD MORE
You're viewed 9 of 10 products
Daicel Pharma synthesizes Raloxifene impurity standards such as Raloxifene Dimer, Raloxifene N-Oxide, 2-(4-hydroxyphenyl)benzo[b]thiophen-5-ol, 4-(3-(piperidin-1-yl) propoxy) benzoic acid hydrochloride (Propyl analogue of PA compound), and more. These impurities are essential for evaluating the quality, stability, and safety of Raloxifene, which is an active pharmaceutical ingredient. Furthermore, Daicel Pharma offers custom synthesis of Raloxifene impurities and delivers them globally.
Raloxifene [CAS: 84449-90-1] belongs to the family of 1-benzothiophenes for treating osteoporosis. It functions as a bone density preserver, an estrogen receptor modulator, and an estrogen antagonist.
Raloxifene is for postmenopausal women to prevent and treat osteoporosis, including corticosteroid-induced bone loss, and to lower the risk of invasive breast cancer in postmenopausal women with osteoporosis or high risk for invasive breast cancer. This drug is available under the brand name Evista.
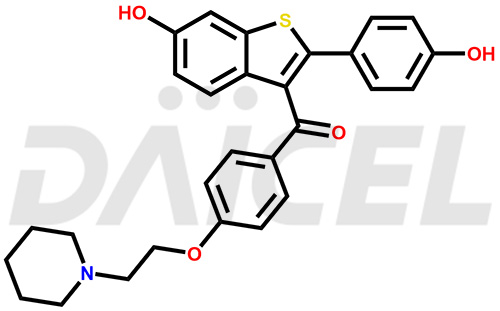
The chemical name of Raloxifene is [6-Hydroxy-2-(4-hydroxyphenyl)benzo[b]thien-3-yl][4-[2-(1-piperidinyl)ethoxy]phenyl]methanone. Its chemical formula is C28H27NO4S, and its molecular weight is approximately 473.6 g/mol.
Raloxifene prevents cytokine production and decreases bone resorption in postmenopausal women.
Raloxifene impurities are chemical compounds that form in addition to the target Raloxifene API during its manufacture1 or storage. They arise from starting materials, reagents, intermediates, or degradation products. Impurity identification and characterization ensure the final Raloxifene product’s quality, safety, and efficacy.
Daicel Pharma offers a Certificate of Analysis (CoA) for Raloxifene impurity standards that include Raloxifene Dimer, Raloxifene N-Oxide, 2-(4-hydroxyphenyl)benzo[b]thiophen-5-ol, 4-(3-(piperidin-1-yl) propoxy) benzoic acid hydrochloride (Propyl analogue of PA compound), and more. Our CoA is from our cGMP-certified analytical laboratory and includes detailed characterization data such as 1H NMR, 13C NMR, IR, MASS, and HPLC purity2. More characterization details, such as those for 13C-DEPT, can be provided on request. Our team of professionals at Daicel Pharma specializes in synthesizing Raloxifene impurities and labeled compounds for testing the efficacy of generic Raloxifene. We provide Raloxifene D4, a deuterium-labeled Raloxifene standard, essential for bioanalytical research and Bioavailability/Bioequivalence (BA/BE) studies.
The presence of impurities in Raloxifene might potentially influence the drug's bioavailability.
Raloxifene impurities can vary between batches and may not be consistent.
Raloxifene impurities can form through various processes such as degradation, side reactions, or incomplete purification during the synthesis or storage of the drug.
Raloxifene impurities are stored at a regulated room temperature of 2-8°C or as specified on the Certificate of Analysis (CoA).
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.