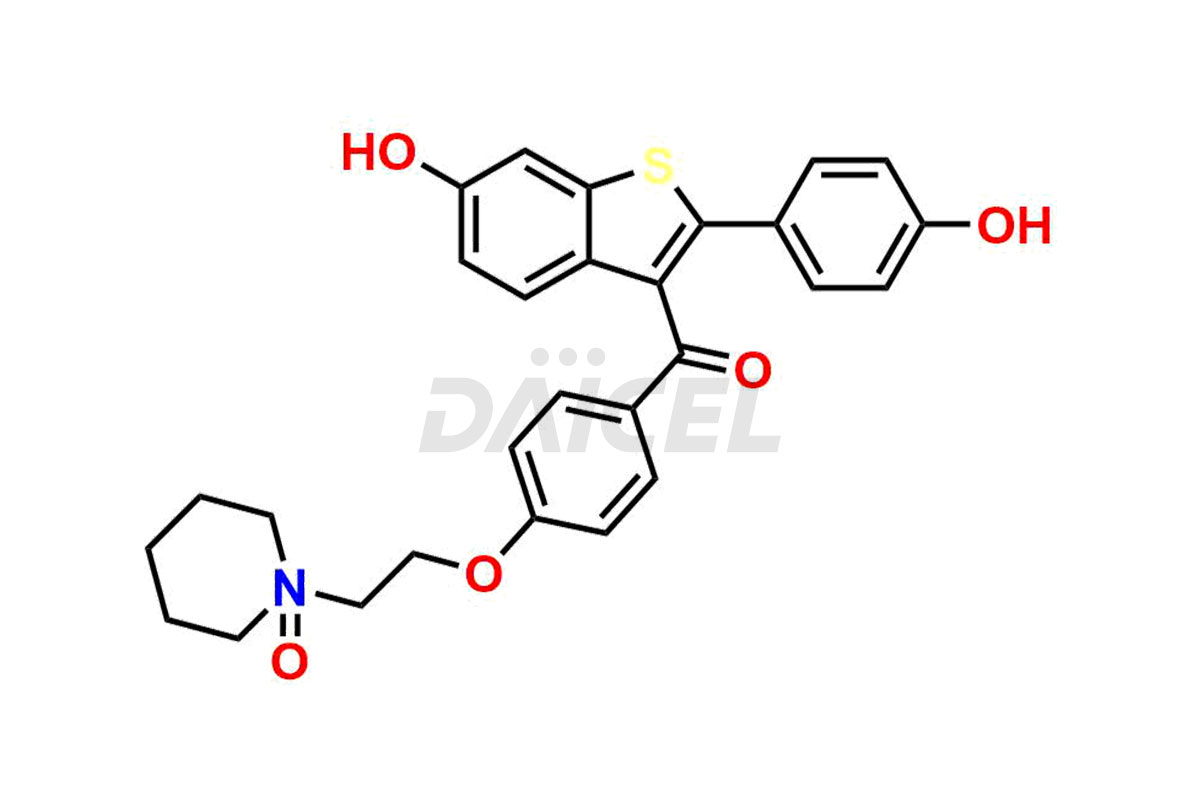
Raloxifene
LOAD MORE
You're viewed all 10 products
References
FAQ's
References
- Jones, Charles David, Benzothiophene compounds and process for preparing them, Eli Lilly and Co., United States, EP0062503A1, October 13, 1982
- Basavaiah, Kanakapura; Kumar, Urdigere Rangachar Anil; Tharpa, Kalsang, Gradient HPLC analysis of raloxifene hydrochloride and its application to drug quality control, Acta Pharmaceutica (Zagreb, Croatia), Volume: 58, Issue: 3, Pages: 347-356, 2008
Frequently Asked Questions
Can Raloxifene impurities influence the drug's bioavailability?
The presence of impurities in Raloxifene might potentially influence the drug's bioavailability.
Are Raloxifene impurities batch-specific or consistent?
Raloxifene impurities can vary between batches and may not be consistent.
How do Raloxifene impurities form?
Raloxifene impurities can form through various processes such as degradation, side reactions, or incomplete purification during the synthesis or storage of the drug.
What are the temperature conditions required to store Raloxifene impurities?
Raloxifene impurities are stored at a regulated room temperature of 2-8°C or as specified on the Certificate of Analysis (CoA).
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.