LOAD MORE
You're viewed 9 of 22 products
Daicel Pharma is capable of offering Pemetrexed impurity standards, such as Pemetrexed EP Imp-E, Pemetrexed Impurity B, Pemetrexed Impurity C, Pemetrexed EP Impurity A, Pemetrexed des-glutamate and more. The impurities can influence the effectiveness, stability, and safety of Pemetrexed. Daicel Pharma can synthesize custom Pemetrexed impurities and deliver them worldwide.
Pemetrexed [CAS: 137281-23-3] is an N-acylglutamic acid compound. It acts as an antineoplastic agent, antimetabolite, and inhibitor of enzymes such as thymidylate synthase, dihydrofolate reductase, and phosphoribosylglycinamide formyltransferase. It treats non-small cell lung cancer and malignant mesothelioma.
Pemetrexed is an FDA-approved anti-folate treatment for malignant pleural mesothelioma and metastatic non-squamous, non-small cell lung cancers. It combines with Pembrolizumab and platinum-based chemotherapy as the initial treatment for metastatic NSCLC without specific genetic aberrations. Further, in combination with cisplatin, Pemetrexed is the first-line therapy for locally advanced or metastatic NSCLC. Pemetrexed is for locally, advanced, or metastatic NSCLC as a maintenance treatment that has not progressed after four cycles of platinum-based chemotherapy. It is for treating recurrent metastatic disease following prior chemotherapy. This medication is available under the trade names Alimta and Pemfexy.
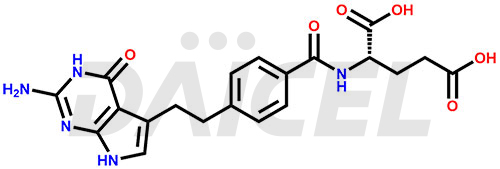
The chemical name of Pemetrexed is N-[4-[2-(2-Amino-4,7-dihydro-4-oxo-3H-pyrrolo[2,3-d]pyrimidin-5-yl)ethyl]benzoyl]-L-glutamic acid. Its chemical formula is C20H21N5O6, and its molecular weight is approximately 427.4 g/mol.
Pemetrexed disrupts folate-dependent metabolic processes essential for cell replication.
Pemetrexed impurities can occur during the synthesis1 due to incomplete reactions, side reactions, contaminants in the starting materials, or reagents utilized. The specific synthesis routes for Pemetrexed impurities might vary depending on the impurities and the reaction conditions.
Daicel Pharma offers a Certificate of Analysis (CoA) for Pemetrexed impurity standards can that are Pemetrexed EP Imp-E, Pemetrexed Impurity B, Pemetrexed Impurity C, Pemetrexed EP Impurity A, Pemetrexed des-glutamate and more. We provide a Certificate of Analysis (CoA) from our cGMP-certified analytical lab, which includes extensive characterization data such as 1H NMR, 13C NMR, IR, MASS, and HPLC purity2. Additional characterization information, including 13C-DEPT, can be provided upon request. Our team at Daicel Pharma specializes in synthesizing Pemetrexed impurities and degradation products.
During the development and validation of analytical methods, potential impurities in Pemetrexed evaluate the specificity, sensitivity, and selectivity to ensure impurities' accurate identification, quantification, and control following regulatory guidelines and standards.
Pemetrexed's stability and shelf life might be affected by impurities, which could eventually influence the drug's effectiveness and dependability.
Pemetrexed impurities are recorded and supported in the medication's specifications through comprehensive analytical testing, validation data, and documentation of impurity profiles, limits, and control measures to ensure compliance with regulatory guidelines.
Pemetrexed impurities are stored at a regulated room temperature of 2-8°C or as specified on the Certificate of Analysis (CoA).
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.