LOAD MORE
You're viewed 9 of 12 products
Daicel Pharma is a trusted provider of quality Metoprolol impurity standards, including 1,3-Bis(isopropylamino)propan-2-ol, Metoprolol Bis propanol, Metoprolol EP Impurity B, Metoprolol EP Impurity F, Metoprolol EP Impurity G, Metoprolol EP Impurity J, Ortho-metoprolol and many more. These impurities are critical in determining the active pharmaceutical ingredient Metoprolol’s quality, stability, and biological safety. Additionally, Daicel Pharma can synthesize Metoprolol impurities according to precise customer specifications while guaranteeing worldwide delivery.
Metoprolol [CAS: 51384-51-1] is a selective beta-blocker widely used to treat hypertension and angina pectoris.
Metoprolol treats angina pectoris, myocardial infarction, heart failure, atrial fibrillation, atrial flutter, and hypertension. It is a selective beta-1 blocker that treats many cardiovascular diseases.
Metoprolol is available under the brand names Kaspargo Sprinkle, Lopressor, and Toprol-XL, which contains the active ingredient Metoprolol.
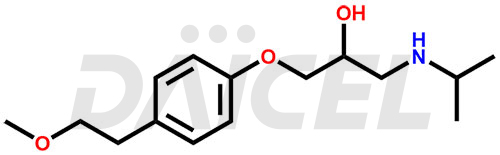
The chemical name of Metoprolol is 1-[4-(2-Methoxyethyl)phenoxy]-3-[(1-methylethyl)amino]-2-propanol. Its chemical formula is C15H25NO3, and its molecular weight is approximately 267.36 g/mol.
Metoprolol blocks beta2-adrenoreceptors located in the bronchial and vascular muscles.
Metoprolol impurities can arise during synthesis1 due to the storage or use of specific raw materials and intermediates in manufacturing. These impurities encompass related compounds, degradation products, and process impurities. Stringent quality control measures and analytical methods are crucial to ensure the purity and safety of Metoprolol for patient use.
Daicel Pharma provides a comprehensive Certificate of Analysis (CoA) for Metoprolol impurity standards, such as 1,3-Bis(isopropylamino)propan-2-ol, Metoprolol Bis propanol, Metoprolol EP Impurity B, Metoprolol EP Impurity F, Metoprolol EP Impurity G, Metoprolol EP Impurity J, Ortho-metoprolol and many more. The CoA includes detailed characterization data such as 1H NMR, 13C NMR, IR, MASS, and HPLC purity2. Additionally, upon delivery, a complete 13C-DEPT is also provided. Daicel possesses the technology and expertise to synthesize any unknown Metoprolol impurity or degradation product. We offer Metoprolol Labelled Standard, deuterium-labeled impurity standards of Metoprolol, which are essential in BA/BE studies.
Analytical Methods like reverse-phase ultra-performance liquid chromatographic method have been developed to estimate Metoprolol impurities quantitatively.
Yes, impurities in Metoprolol can impact patient safety. Depending on their nature and concentration, contaminants can cause adverse effects or reduce the efficacy of the medication.
Methanol is employed to attain the ideal solubility and differentiation of impurities in Metoprolol.
Metoprolol impurities should generally be stored at a controlled room temperature between 2-8 °C or as indicated on the Certificate of Analysis (CoA).
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.