LOAD MORE
You're viewed 9 of 12 products
For evaluating the purity and safety of Macitentan, an essential active pharmaceutical ingredient, Daicel Pharma offers a customized synthesis of Macitentan impurity standards. These impurity standards include crucial compounds such as HN-MCTRCO1, HN-MCTRCO2, N-Despropyl Macitentan, HN-MCTRCO3, and more. Additionally, Daicel Pharma provides worldwide delivery options for Macitentan impurity standards.
Macitentan [CAS: 441798-33-0] has antihypertensive and antineoplastic properties and is a dual endothelin receptor (ETR) antagonist. It treats pulmonary arterial hypertension (PAH). Acting as an endothelin receptor antagonist, Macitentan functions as an antihypertensive agent by blocking the effects of endothelin, a hormone involved in blood vessel constriction.
Opsumit, which contains the active ingredient Macitentan, is a medication for the long-term treatment of patients with pulmonary arterial hypertension (PAH) classified as World Health Organization (WHO) Functional Class II or III. It reduces morbidity in PAH cases associated with idiopathic or heritable factors and those linked to connective tissue disease or congenital heart disease. Opsumit can be used alone or in combination with phosphodiesterase-5 (PDE-5) inhibitors as a part of the treatment regimen.
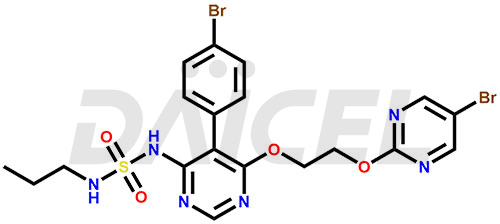
The chemical name of Macitentan is N-[5-(4-Bromophenyl)-6-[2-[(5-bromo-2-pyrimidinyl)oxy]ethoxy]-4-pyrimidinyl]-N′-propylsulfamide. Its chemical formula is C19H20Br2N6O4S, and its molecular weight is approximately 588.3 g/mol.
Macitentan prevents the binding of Endothelin (ET)-1 to ETA and ETB receptors in smooth muscle cells.
The analysis and control of impurities in Macitentan1, an endothelin receptor antagonist used for pulmonary arterial hypertension, are critical for ensuring its safety and efficacy. Impurities in Macitentan may include related compounds and degradation products. Analytical techniques such as high-performance liquid chromatography (HPLC) and mass spectrometry (MS) help identify and quantify these impurities. Stringent control measures and specifications limit the presence of impurities in Macitentan, following regulatory guidelines.
Daicel Pharma strictly adheres to cGMP standards and operates an analytical facility for preparing Macitentan impurity standards, which include HN-MCTRCO1, HN-MCTRCO2, N-Despropyl Macitentan, HN-MCTRCO3, and more. In addition, we offer deuterium-labeled Macitentan standard, Macitentan-D4, which is essential for conducting bioanalytical research and BA/BE studies. Our Macitentan impurity standards have a detailed Certificate of Analysis (CoA) that provides a comprehensive characterization report. This report includes data obtained through techniques, 1H NMR, 13C NMR, IR, MASS, and HPLC purity analysis2. Upon request, we give additional data like 13C-DEPT. Moreover, we can synthesize unknown Macitentan impurity standards, degradation products, and labeled compounds to evaluate the effectiveness of generic Macitentan. Each delivery has a comprehensive characterization report.
Regulatory authorities require manufacturers to report and document the presence of impurities in Macitentan formulations, including their identification, quantification, and compliance with specified limits.
Some impurities in Macitentan can contribute to changes in color, odor, or taste. Strict control measures are in place to ensure the product's organoleptic properties remain within acceptable limits.
Acetonitrile is the solvent used when analyzing many impurities in Macitentan.
Macitentan impurities should be stored, at a controlled room temperature, usually between 2-8 °C.
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.