LOAD MORE
You're viewed 9 of 35 products
Daicel Pharma offers worldwide delivery options for a custom synthesis of Ibuprofen impurities, including impurities such as Ibuprofen Ph. Eur. Impurity G, Ibuprofen Unknown Impurity, 3-Hydroxy-2,2-dimethylpropyl 2-(4-isobutylphenyl)propanoate, Ibuprofen 1,2-Propylene Glycol Esters (Mixture of Regio and Stereo isomers), Ibuprofen 1,2,3-propanetriol esters (mixture of regio and stereo isomers), and so on. These impurities play a vital role in evaluating the purity and safety of Ibuprofen, an active pharmaceutical ingredient.
Ibuprofen [CAS: 15687-27-1], a nonsteroidal anti-inflammatory drug (NSAID) and a propionic acid derivative helps alleviate symptoms related to arthritis, primary dysmenorrhea, and fever. It exhibits analgesic, anti-inflammatory, and antipyretic effects.
Ibuprofen, available under various trademarks, Advil, Caldolor, Ibuprohm, Medipren, Nuprin, etc., is a nonsteroidal anti-inflammatory drug (NSAID). It manages and treats inflammatory diseases, rheumatoid disorders, mild to moderate pain, fever, dysmenorrhea, and osteoarthritis. It is available by prescription for more severe conditions and over the counter for mild pain relief.
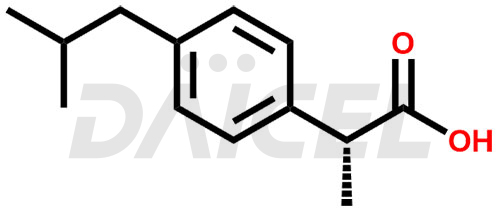
The chemical name of Ibuprofen is α-Methyl-4-(2-methylpropyl) benzene acetic acid. Its chemical formula is C13H18O2, and its molecular weight is approximately 206.28 g/mol.
Ibuprofen inhibits prostaglandin precursors, such as cyclooxygenase, an enzyme for prostaglandin synthesis.
Ibuprofen can contain impurities that arise from manufacturing processes1 or storage. The common Ibuprofen impurities include Ibuprofen isomer impurities, related carboxylic acid impurities, organic impurities from starting materials or intermediates, inorganic impurities such as heavy metals or salts, and residual solvents used during synthesis or purification. Regulatory authorities set limits on impurity levels to ensure the safety and quality of Ibuprofen. Manufacturers adhere to strict quality control measures to minimize impurities and meet regulatory standards for the final product.
Daicel Pharma strictly adheres to cGMP standards and operates an analytical facility for the preparation of Ibuprofen impurity standards, such as Ibuprofen Ph. Eur. Impurity G, Ibuprofen Unknown Impurity, 3-Hydroxy-2,2-dimethylpropyl 2-(4-isobutylphenyl)propanoate, Ibuprofen 1,2-Propylene Glycol Esters (Mixture of Regio and Stereo isomers), Ibuprofen 1,2,3-propanetriol esters (mixture of regio and stereo isomers), and so on. We offer deuterium-labeled Ibuprofen compounds, including Ibuprofen-D3, Ibuprofen-D3 S-isomer, Ibuprofen-13CD3, and Ibuprofen-D3 R-isomer, essential for bioanalytical research and BA/BE studies. Our impurities have a detailed Certificate of Analysis (CoA) that provides a comprehensive characterization report. This report includes data obtained through techniques, 1H NMR, 13C NMR, IR, MASS, and HPLC purity analysis2. Upon request, we give additional data like 13C-DEPT. We can synthesize unknown Ibuprofen impurities or degradation products and labeled compounds. Each delivery has a comprehensive characterization report.
If the presence of impurities in Ibuprofen is within acceptable limits, there is no cause for adverse effects or its impact on effectiveness. However, higher impurity levels could affect the drug's safety and efficacy.
While different brands of Ibuprofen may vary in manufacturing processes and quality control measures, all formulations must comply with regulatory standards for impurity levels. Therefore, the presence of impurities should be within acceptable limits regardless of the brand.
Acetonitrile or Methanol are the commonly used solvents when analyzing many impurities in Ibuprofen.
The recommendation is to store Ibuprofen impurities at a controlled room temperature, within 2-8 °C.
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.