LOAD MORE
You're viewed 9 of 17 products
Daicel Pharma synthesizes more than fifteen high-quality Ibrutinib impurities, such as (S)-Ibrutinib, 4-Hydroxy Ibrutinib, IBR Diamine Impurity, Ibrutinib acetyl impurity, Ibrutinib amine impurity, Ibrutinib chloro impurity, Ibrutinib methoxy impurity, IBT6A adduct and more, crucial in determining the quality, stability, and biological safety of the active pharmaceutical ingredient, Ibrutinib. Moreover, Daicel Pharma offers custom synthesis of Ibrutinib impurities and delivers them globally.
Ibrutinib [CAS: 936563-96-1] is a medication used to treat B-cell malignancies by selectively inhibiting the enzyme Bruton’s tyrosine kinase (BTK). It is an antineoplastic agent. It is under development for treating various hematological malignancies.
Ibrutinib treats various conditions in adult patients, including Waldenström’s macroglobulinemia, chronic lymphocytic leukemia or small lymphocytic lymphoma, mantle cell lymphoma, and marginal zone lymphoma in patients who require systemic therapy and have received at least one prior anti-CD20-based therapy. It also treats chronic graft-versus-host disease in patients after previous failure of one or more lines of systemic therapy. The drug is available as capsules and tablets under the trade name Imbruvica.
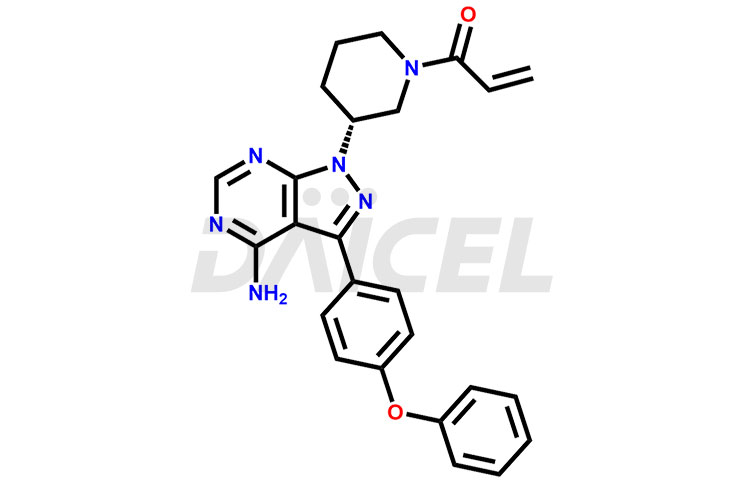
The chemical name of Ibrutinib is 1-[(3R)-3-[4-Amino-3-(4-phenoxyphenyl)-1H-pyrazolo[3,4-d]pyrimidin-1-yl]-1-piperidinyl]-2-propen-1-one. It’s chemical formula is C25H24N6O2 and its molecular weight is approximately 440.5 g/mol.
Ibrutinib is a small molecule inhibitor that targets Bruton’s tyrosine kinase (BTK). It works by binding covalently to a cysteine residue in the BTK active site leading to the inhibition of BTK enzymatic activity. BTK plays a signaling role in B-cell antigen receptor (BCR) and cytokine receptor pathways, essential for B-cell trafficking, chemotaxis, and adhesion.
Ibrutinib impurities are of three types, process-related, degradation, and miscellaneous. Process-related impurities are formed during the synthetic1 and purification process and can include reagents, by-products, and unreacted starting materials. Degradation impurities2 result from the instability of Ibrutinib during storage or under specific conditions such as heat, light, or moisture. Miscellaneous impurities do not fit into the other categories, such as inorganic salts or residual solvents. An example of a process-related impurity is N-Desmethyl Ibrutinib, while a hydrolyzed product of Ibrutinib is a degradation impurity. Impurity presence can impact drug safety, quality, and effectiveness. So to minimize impurities during Ibrutinib synthesis and purification, a thorough analysis and characterization of the final product are necessary to ensure drug safety and efficacy.
Daicel offers a Certificate of Analysis (CoA) from a cGMP-compliant analytical facility for more than fifteen Ibrutinib impurity standards, including (S)-Ibrutinib, 4-Hydroxy Ibrutinib, IBR Diamine Impurity, Ibrutinib acetyl impurity, Ibrutinib amine impurity, Ibrutinib chloro impurity, Ibrutinib methoxy impurity, IBT6A adduct, and more. The CoA includes complete characterization data, such as 1H NMR, 13C NMR, IR, MASS3, and HPLC purity. We also provide 13C-DEPT and CHN on request. We also give a complete characterization report on delivery.
Daicel has the technology and expertise to prepare any unknown Ibrutinib impurity or degradation product. The company also provides labeled compounds to quantify the efficacy of generic Ibrutinib. Daicel offers highly pure brutinib Metabolite-PCI-45227 – D5, Ibrutinib-D5, which are deuterium-labeled standards of Ibrutinib for bioanalytical research and BA/BE studies with the percentage of isotope data in CoA.
Isomeric impurities in Ibrutinib occur due to the presence of stereoisomers during the synthetic process.
Degradant impurities in Ibrutinib result from the breakdown of the drug substance due to factors such as hydrolysis, oxidation, or photolysis.
The residual solvents in Ibrutinib are the solvents used during the synthetic process but remain in the final product in small amounts.
The impurities in Ibrutinib are identified and characterized through analytical methods such as liquid chromatography-mass spectrometry (LC-MS), etc.
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.