LOAD MORE
You're viewed 9 of 12 products
Daicel Pharma offers high-quality impurities for Ethinylestradiol, an active pharmaceutical ingredient. These impurities, including Ethinyl estradiol Impurity H, Ethinyl Estradiol EP Impurity F, Ethinyl estradiol Impurity, Ethinyl estradiol Impurity I, Ethinyl Estradiol Impurity-K, Ethinyl Estradiol Impurity M, Ethinylestradiol Impurity Dimer and so on, play a vital role in assessing the purity, reliability, and safety of Ethinylestradiol. Daicel Pharma also offers a customized synthesis of Ethinylestradiol impurities to cater to client requirements, with worldwide delivery options available.
Ethinyl estradiol [CAS: 57-63-6] is a semisynthetic estrogen. It is a modified form of estradiol with a 17-alpha-Ethinyl substitution. It exhibits high estrogenic potency and is the estrogen component in oral contraceptives. Additionally, Ethinyl estradiol may delay the progress of prostate cancer.
Ethinyl estradiol, a synthetic estrogen, is widely available under various brand names such as Alesse, Tri-Cyclen, Triphasil, etc., as a combination oral contraceptive. When combined with a progestogen, Ethinyl estradiol is used as an oral contraceptive to prevent pregnancy, with FDA approval. It also protects against osteoporosis and may delay the progress of prostate cancer.
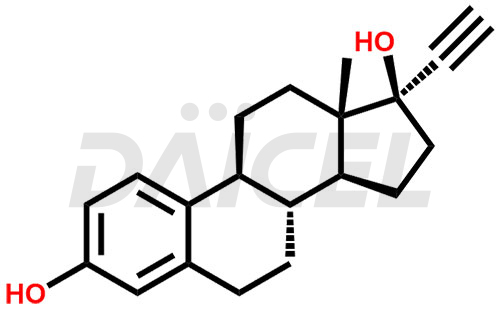
The chemical name of Ethinylestradiol is (17α)-19-Norpregna-1,3,5(10)-trien-20-yne-3,17-diol. Its chemical formula is C20H24O2, and its molecular weight is approximately 296.4 g/mol.
Ethinylestradiol acts by binding to the estrogen receptor complex and entering the nucleus. It triggers the activation of DNA transcription, leading to gene expression in estrogenic cellular responses. It inhibits 5-alpha reductase in epididymal tissue, lowers testosterone levels, and delays the progress of prostate cancer.
Impurities in Ethinylestradiol are unintended substances or byproducts that can be present in the medication. They can emerge during the synthesis1, manufacturing, or storage processes. They may include related compounds, degradation products, residual solvents, or other impurities introduced during synthesis or handling. The presence of impurities can impact Ethinylestradiol quality, stability, and effectiveness. Strict regulatory guidelines and quality control measures help monitor and control the levels of impurities in Ethinylestradiol.
Daicel Pharma offers a comprehensive Certificate of Analysis (CoA) for Ethinylestradiol impurity standards, including Ethinyl estradiol Impurity H, Ethinyl Estradiol EP Impurity F, Ethinyl estradiol Impurity, Ethinyl estradiol Impurity I, Ethinyl Estradiol Impurity-K, Ethinyl Estradiol Impurity M, Ethinylestradiol Impurity Dimer and so on. These impurities are produced by an analytical facility that complies with cGMP standards. The CoA provides a detailed characterization report with data obtained through techniques such as 1H NMR, 13C NMR, IR, MASS, and HPLC purity analysis2. We give additional data like 13C-DEPT upon request. Daicel Pharma synthesizes unknown Ethinylestradiol impurities or degradation products. Every delivery has a complete characterization report.
Impurities in Ethinylestradiol are identified through comparison with reference standards, spectral analysis, and mass spectrometry to determine their chemical structure and composition.
Stability testing helps monitor impurity levels in Ethinylestradiol formulations during their shelf life to ensure product quality and safety.
Genotoxicity tests help evaluate the potential DNA-damaging effects of impurities in Ethinylestradiol formulations.
Ethinylestradiol impurities are stored at a controlled room temperature, 2-8 °C or according to the Certificate of Analysis (CoA) specifications.
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.