LOAD MORE
You're viewed 9 of 20 products
Daicel Pharma offers high-quality impurities for Eliglustat, an active pharmaceutical ingredient. These impurities, including (1R, 2S) Eliglustat Hemi Tartaric Salt, (1S, 2R) Eliglustat Hemi Tartaric Salt, (1S, 2S)-Eliglustat, Eliglustat Impurity A, Eliglustat N-Oxide, Eliglustat O-Octanyl Impurity, and more, play a vital role in assessing the purity, reliability, and safety of Eliglustat. Daicel Pharma also offers a customized synthesis of Eliglustat impurities to cater to client requirements, with worldwide delivery options available.
Eliglustat [CAS: 491833-29-5] is an orally available glucosylceramide synthase (GCS) inhibitor for treating type 1 Gaucher disease. It decreases the production of glucosylceramide and other GSLs that affect immune processes and functions.
Eliglustat, available under Cerdelga, is a small molecule inhibitor of glucosylceramide synthase and is the rate-controlling step in glycolipid synthesis. By inhibiting this pathway, the levels of glycolipid substrates decrease, leading to reduced lysosomal degradation and accumulation of glycosylceramide.
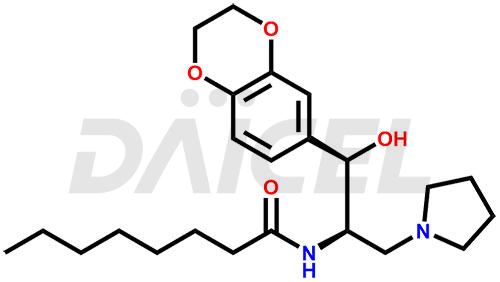
The chemical name of Eliglustat is N-[(1R,2R)-2-(2,3-Dihydro-1,4-benzodioxin-6-yl)-2-hydroxy-1-(1-pyrrolidinylmethyl)ethyl]octanamide. Its chemical formula is C23H36N2O4, and its molecular weight is approximately 404.5 g/mol.
Eliglustat inhibits glucosylceramide synthase and acts as a substrate-reduction therapy for Gaucher 4 disease type 1 (GD1).
The thorough examination and control of impurities in Eliglustat, a medication to treat type 1 Gaucher disease, are vital to ensure its safety and effectiveness. Various analytical techniques, such as chromatography, spectroscopy, and mass spectrometry, are employed to identify and measure impurities in Eliglustat. The process of impurity profiling helps in understanding their chemical composition, structure, and potential risks associated with them in the drug. Stringent measures during manufacturing1 help minimize the formation of impurities. Regulatory guidelines set specific limits for Eliglustat impurities in pharmaceutical products, and their comprehensive analysis and control play a crucial role in maintaining high quality and therapeutic value for patients.
Daicel Pharma offers a comprehensive Certificate of Analysis (CoA) for Eliglustat impurity standards, including (1R, 2S) Eliglustat Hemi Tartaric Salt, (1S, 2R) Eliglustat Hemi Tartaric Salt, (1S, 2S)-Eliglustat, Eliglustat Impurity A, Eliglustat N-Oxide, Eliglustat O-Octanyl Impurity,
and more. They generate from an analytical facility that complies with cGMP standards. The CoA provides a detailed characterization report with data obtained through techniques such as 1H NMR, 13C NMR, IR, MASS, and HPLC purity analysis2. We provide additional data like 13C-DEPT upon request. Daicel Pharma synthesizes unknown Eliglustat impurities or degradation products, and labeled compounds, to evaluate the efficacy of generic Eliglustat. Eliglustat – D15, a deuterium-labeled Eliglustat standard, is available for bio-analytical research, including BA/BE studies. Every delivery has a complete characterization report.
The formation of Eliglustat impurities is minimized by optimizing reaction conditions, employing suitable purification techniques, ensuring proper storage and handling, and conducting stability studies to understand degradation pathways.
Impurities in Eliglustat can potentially impact its efficacy by altering the drug's pharmacokinetics, stability, or interaction with the target receptors. Hence, their control is essential to maintain the desired therapeutic effect.
Analyzing Eliglustat impurities can be challenging due to their low concentrations, potential complexity, and the need for sensitive and specific analytical techniques. Validation of analytical methods is crucial for accurate impurity detection.
Eliglustat impurities are stored at a controlled room temperature, 2-8 °C, or under a Nitrogen atmosphere. You can refer to the storage on the Certificate of Analysis (CoA) specifications.
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.