LOAD MORE
You're viewed 9 of 21 products
Daicel Pharma specializes in offering high-quality impurities for Efinaconazole, an active pharmaceutical ingredient. These impurities, including (2S,3S) Efinaconazole Enantiomer, 1-(2-bromo-5-fluorophenyl)-1H-1,2,4-triazole, 2-Fluoro Efinaconazole, and more, play a vital role in assessing the purity, reliability, and safety of Efinaconazole. Daicel Pharma also offers a customized synthesis of Efinaconazole impurities to cater to client requirements, with worldwide delivery options available.
Efinaconazole [CAS: 164650-44-6] is a triazole antifungal for treating onychomycosis of the toenails due to Trichophyton rubrum and Trichophyton mentagrophytes.
Efinaconazole, available under Jublia, is a triazole compound with broad-spectrum antifungal activity. It treats onychomycosis and other superficial fungal infections. Efinaconazole has shown effectiveness against various fungal species, including Candida and Aspergillus. The therapeutic use of Efinaconazole may extend beyond onychomycosis in the future.
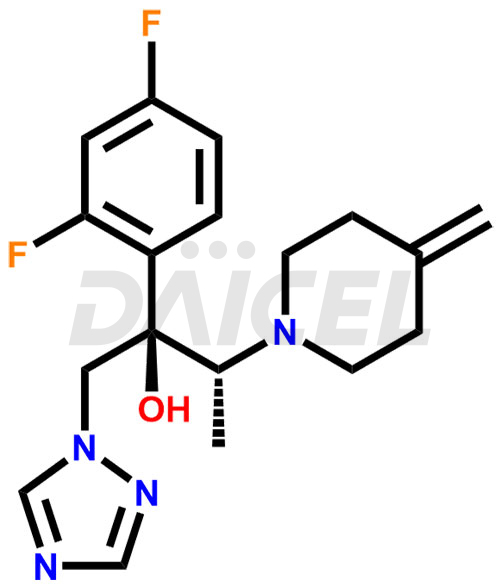
The chemical name of Efinaconazole is (αR, βR)-α-(2,4-Difluorophenyl)-β-methyl-4-methylene-α-(1H-1,2,4-triazol-1-ylmethyl)-1-piperidineethanol. Its chemical formula is C18H22F2N4O, and its molecular weight is approximately 348.4 g/mol.
Efinaconazole inhibits fungal lanosterol 14α-demethylase, which causes the biosynthesis of ergosterol, a part of fungal cell membranes.
The analysis and control of impurities in Efinaconazole, a pharmaceutical compound, are critical to ensure its quality and safety. Throughout the synthesis1, storage, or degradation of Efinaconazole, impurities generate, which can affect its efficacy or pose potential risks. The thorough analysis identifies and quantifies these impurities, allowing control within acceptable limits. Strict control measures during the manufacturing process help minimize impurity levels and maintain the desired quality of Efinaconazole. It includes adherence to appropriate storage conditions, rigorous manufacturing practices, and regular quality assessments to uphold the safety and effectiveness of the final product.
Daicel Pharma offers a comprehensive Certificate of Analysis (CoA) for Efinaconazole impurity standards, including (2S,3S) Efinaconazole Enantiomer, 1-(2-bromo-5-fluorophenyl)-1H-1,2,4-triazole, 2-Fluoro Efinaconazole, and more. They generate from an analytical facility that complies with cGMP standards. The CoA provides a detailed characterization report with data obtained through techniques such as 1H NMR, 13C NMR, IR, MASS, and HPLC purity analysis2. We provide additional data like 13C-DEPT upon request. Daicel Pharma synthesizes unknown Efinaconazole impurities or degradation products. Every delivery has a complete characterization report.
Yes, impurities in Efinaconazole undergo comprehensive toxicological evaluations to determine their potential toxicity and establish safe exposure limits. This assessment ensures patient safety during drug administration.
Some impurities in Efinaconazole can impact its stability over time, potentially leading to degradation or reduced shelf life. Stability studies assess the drug's integrity under various storage conditions.
Impurity profiles for Efinaconazole are regularly reviewed and updated to align with evolving regulatory guidelines, scientific advancements, and emerging safety concerns. These updates help ensure the drug's continued quality and safety.
Efinaconazole impurities are stored at a controlled room temperature between 2-8 °C or as indicated on the Certificate of Analysis (CoA).
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.