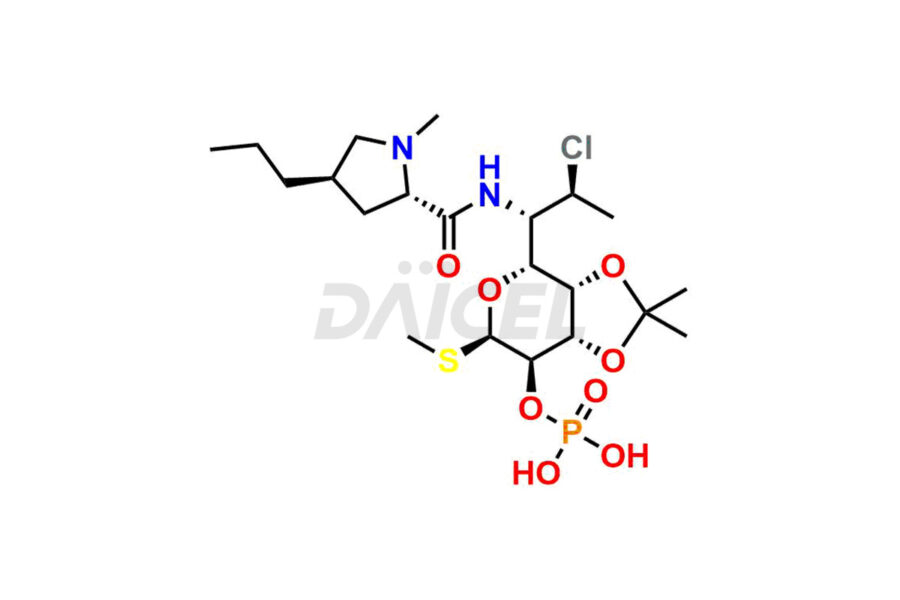
Clindamycin
References
- Birkenmeyer, Robert D.; Kagan, Fred, Sulfoxides Of 7-Halo-7-Deoxylincomycins And Process, Upjohn Co., US3513155A, May 19, 1970
- Landis, J. B.; Grant, M. E.; Nelson, S. A., Determination of clindamycin in pharmaceuticals by high-performance liquid chromatography using ion-pair formation, Journal of Chromatography, Volume: 202, Issue: 1, Pages: 99-106, 1980
Frequently Asked Questions
How are Clindamycin impurities characterized?
Clindamycin impurities are characterized using various analytical techniques, including spectroscopic methods (IR, UV), mass spectrometry (MS), and comparison with reference standards.
What is the role of stability studies in evaluating Clindamycin impurities?
Stability studies help assess the formation and degradation of Clindamycin impurities over time under various storage conditions, providing valuable information for impurity control strategies.
Which solvent helps in the analysis of Clindamycin impurities?
Acetonitrile is a solvent used in analyzing many impurities in Clindamycin.
What are the temperature conditions required to store Clindamycin impurities?
Clindamycin impurities are stored at a controlled room temperature between 2-8 °C or as indicated on the Certificate of Analysis (CoA).
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.