LOAD MORE
You're viewed 9 of 10 products
Daicel Pharma synthesizes Clindamycin impurities of exceptional quality, such as Clindamycin Imp C, Clindamycin impurity D, Clindamycin impurity E, Clindamycin Impurity F, Clindamycin phosphate sulfoxide, Isopropylidene Clindamycin, and Isopropylidene Clindamycin Phosphate. These impurities are crucial to assess the purity, reliability, and safety of an active pharmaceutical ingredient, Clindamycin. Besides, Daicel Pharma provides custom synthesis of Clindamycin impurities to meet clients’ demands for global delivery.
Clindamycin [CAS: 18323-44-9] is an antibiotic and a lincomycin derivative. It has a broad spectrum of activity against various bacterial infections and is bacteriostatic.
Clindamycin treats various infections, including septicemia, intra-abdominal infections, lower respiratory infections, gynecological infections, bone and joint infections, and skin and skin structure infections. It treats streptococcal pharyngitis, acne vulgaris, bacterial vaginosis, severe pelvic inflammatory disease, and certain types of pneumonia. Furthermore, Clindamycin shows effectiveness against conditions like babesiosis, anthrax, malaria, and uncomplicated skin and soft tissue infections, particularly those caused by methicillin-resistant Staphylococcus aureus (MRSA). Clindamycin can be administered orally, topically, or parenterally, depending on the type and severity of the infection. Various brand names, including Cleocin, Clindagel, Clindesse, Clindets, Evoclin, Veltin, Xaciato, etc., are available in different formulations.
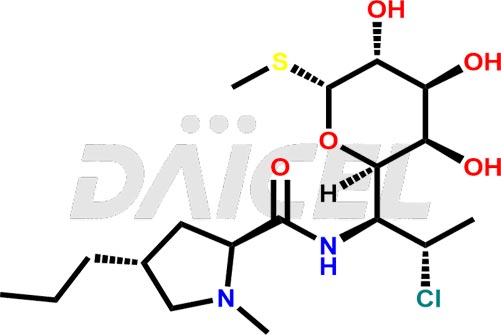
The chemical name of Clindamycin is Methyl 7-chloro-6,7,8-trideoxy-6-[[[(2S,4R)-1-methyl-4-propyl-2-pyrrolidinyl]carbonyl]amino]-1-thio-L-threo-α-D-galacto-octopyranoside. Its chemical formula is C18H33ClN2O5S, and its molecular weight is approximately 425.0 g/mol.
Clindamycin inhibits bacterial protein synthesis. It binds to the 23S RNA of the 50S subunit of the ribosome.
Clindamycin is a commonly used antibiotic, and its impurities can arise from various sources, including the synthetic process1, degradation during storage, and contaminants in raw materials. They affect the drug’s safety, efficacy, and quality. Therefore, it is necessary to identify and quantify these impurities through analytical testing and implement appropriate measures to control their levels. Common Clindamycin impurities include related substances, degradation products, and residual solvents. Analytical methods such as HPLC and LC help analyze and monitor these impurities.
Daicel Pharma offers a Certificate of Analysis (CoA) for Clindamycin impurity standards, such as Clindamycin Imp C, Clindamycin impurity D, Clindamycin impurity E, Clindamycin Impurity F, Clindamycin phosphate sulfoxide, Isopropylidene Clindamycin, and Isopropylidene Clindamycin Phosphate, generated from an analytical facility compliant with cGMP standards. The CoA includes a comprehensive characterization report comprising data from techniques like 1H NMR, 13C NMR, IR, MASS, and HPLC purity2. Furthermore, on request, we give additional data like 13C-DEPT and CHN. Daicel Pharma can synthesize unknown Clindamycin impurities or degradation products. A complete characterization report accompanies every delivery.
Clindamycin impurities are characterized using various analytical techniques, including spectroscopic methods (IR, UV), mass spectrometry (MS), and comparison with reference standards.
Stability studies help assess the formation and degradation of Clindamycin impurities over time under various storage conditions, providing valuable information for impurity control strategies.
Acetonitrile is a solvent used in analyzing many impurities in Clindamycin.
Clindamycin impurities are stored at a controlled room temperature between 2-8 °C or as indicated on the Certificate of Analysis (CoA).
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.