LOAD MORE
You're viewed 9 of 14 products
Daicel Pharma synthesizes more than ten high-quality Cisatracurium impurities, including CIS HPOCE-R-LAUDA Trifluoroacetate, Cis-Cis-triester anolog, Cisatracurium EP impurity-B, Cisatracurium EP impurity-D, Cisatracurium EP impurity-E, Cisatracurium EP impurity-F, Cisatracurium EP impurity-N, Cisatracurium EP Impurity-O, Cisatracurium EP impurity-R, Cisatracurium EP impurity-W, CIS-CE-RLAUDANOSINE BESYLATE Cp 93242 , and more, which are crucial in the analysis of the quality, stability, and biological safety of the active pharmaceutical ingredient, Cisatracurium. Moreover, Daicel Pharma offers custom synthesis of Cisatracurium impurities and delivers them globally.
Cisatracurium [CAS: 96946-41-7] or Cisatracurium besylate [CAS: 96946-42-8] is a medicine used during surgery to relax skeletal muscles and help with tracheal intubation. It belongs to a class of drugs called neuromuscular blocking agents and has a benzylisoquinolinium structure. Cisatracurium is similar to another drug called atracurium. It is an intermediate-acting drug, as its effects last for moderate amount of time.
Cisatracurium is commonly used as an adjunct to general anesthesia during surgery in adults and pediatric patients aged one month to 12 years. Additionally, it can provide skeletal muscle relaxation during surgical procedures or mechanical ventilation in adult patients. For pediatric patients aged 2 years and older, Cisatracurium can be administered via infusion to provide skeletal muscle relaxation during surgical procedures. Cisatracurium is available as an injection under the brand name Nimbex.
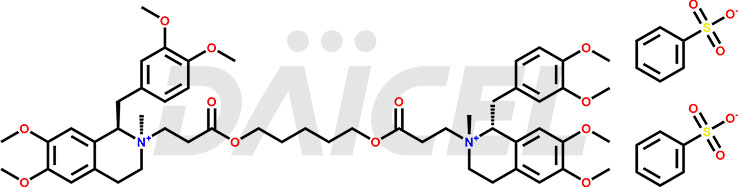
The chemical structure of Cisatracurium is (1R,1′R,2R,2′R)-2,2′-[1,5-Pentanediylbis[oxy(3-oxo-3,1-propanediyl)]]bis[1-[(3,4-dimethoxyphenyl)methyl]-1,2,3,4-tetrahydro-6,7-dimethoxy-2-methylisoquinolinium], Its chemical formula is C53H72N2O12, and its molecular weight is approximately 929.1 g/mol.
Cisatracurium besylate binds competitively to cholinergic receptors, blocking neuromuscular transmission.
The impurities in Cisatracurium besylate form during synthesis due to side reactions or incomplete purification. Strict quality control measures and purification techniques are maintained during the synthesis1 and production of Cisatracurium (Cisatracurium besylate) to minimize the formation of impurities.
Daicel offers a Certificate of Analysis (CoA) from a cGMP-compliant analytical facility for Cisatracurium impurity standards, such as CIS HPOCE-R-LAUDA Trifluoroacetate, Cis-Cis-triester analog, Cisatracurium EP impurity-B, Cisatracurium EP impurity-D, Cisatracurium EP impurity-E, Cisatracurium EP impurity-F, Cisatracurium EP impurity-N, Cisatracurium EP Impurity-O, Cisatracurium EP impurity-R, Cisatracurium EP impurity-W, CIS-CE-RLAUDANOSINE BESYLATE Cp 93242, and so on. The CoA includes complete characterization data, such as 1H NMR, 13C NMR, IR, MASS, and HPLC purity2,3. We also provide 13C-DEPT and CHN on request. We give a complete characterization report on delivery.
Daicel has the technology and expertise to prepare any unknown Cisatracurium impurity or degradation product. We also provide labeled compounds to quantify the efficacy of generic Cisatracurium. Daicel offers highly pure isotope-labeled standards of Cisatracurium for bioanalytical research and BA/BE studies with isotope data in CoA.
Synthesizing Cisatracurium impurities helps identify and quantify the impurities present in Cisatracurium besylate, a neuromuscular blocking agent used during surgery. It is essential for quality control and ensuring the safety and efficacy of the drug.
The detection and quantification of Cisatracurium impurities typically involve analytical techniques such as high-performance liquid chromatography (HPLC), liquid chromatography-mass spectrometry (LC-MS), etc.
Methanol, DCM (Dichloromethane), or DMSO (Dimethyl sulfoxide) are the solvents used based on the impurity profile of Cisatracurium and its impurities.
Cisatracurium impurities storage is at a controlled room temperature between 2-8 ⁰C. However, impurities such as Cisatracurium Besylate EP Impurity-Q are stored at -20 ⁰C as per the stability of the compound.
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.