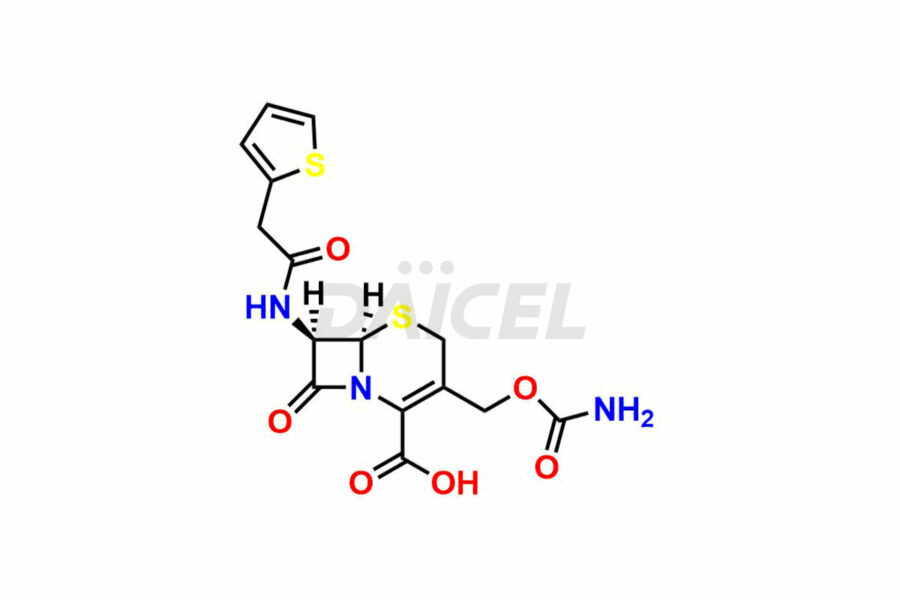
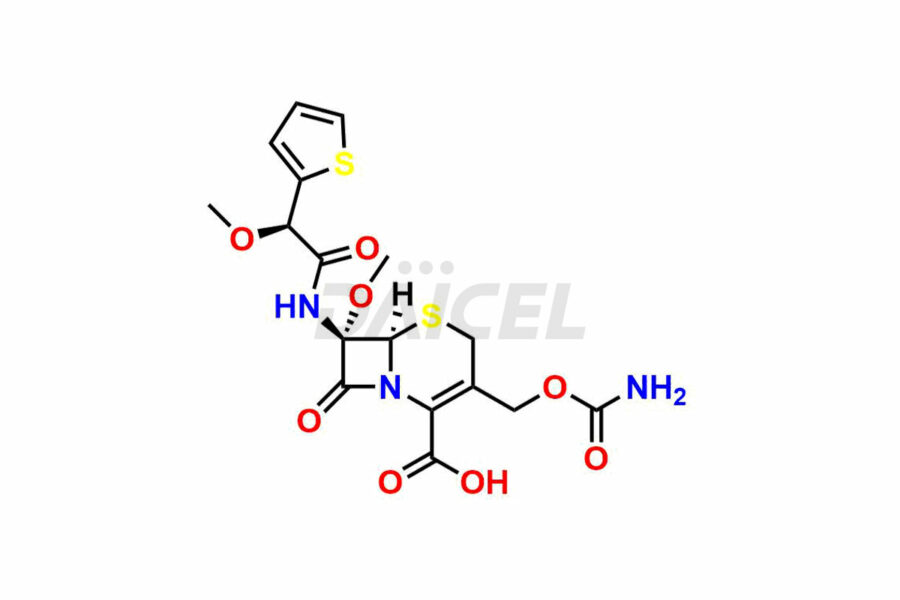
Cefoxitin
General Information
Cefoxitin Impurities and Cefoxitin
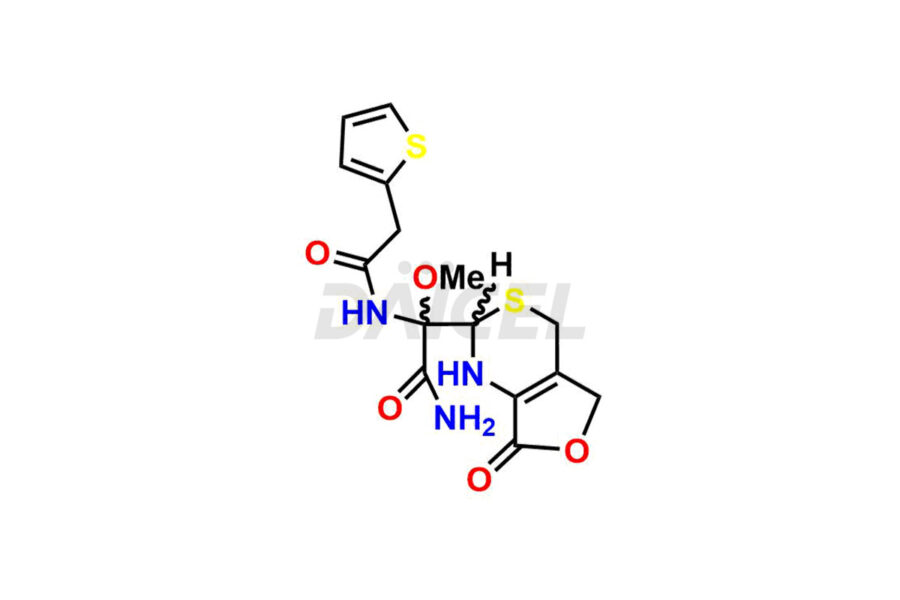
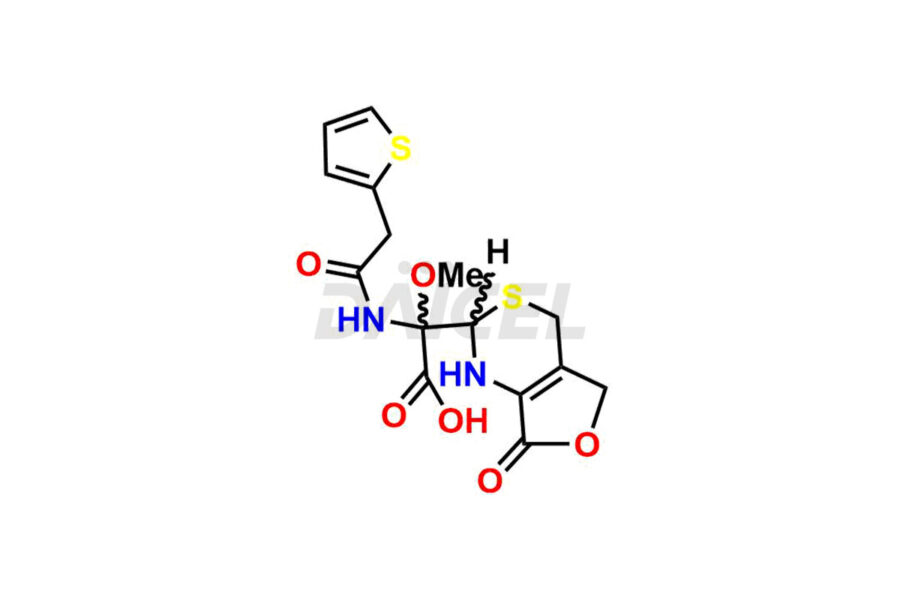
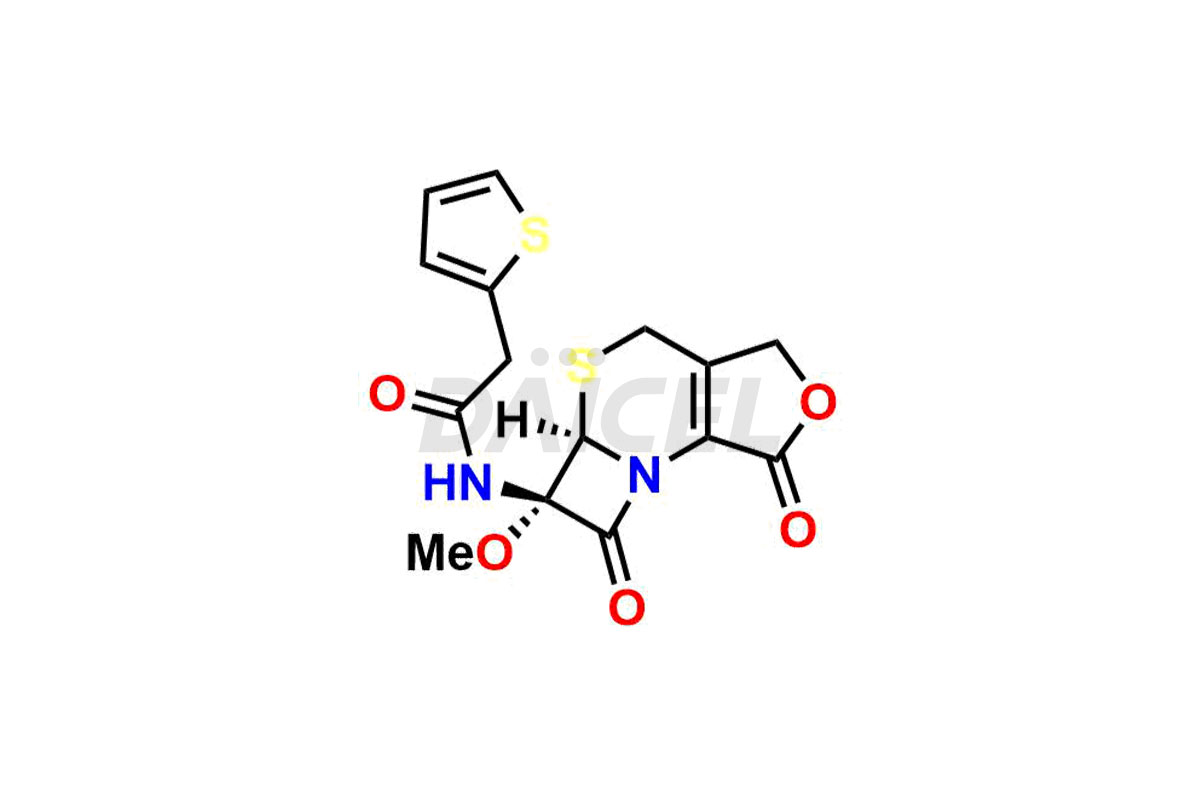
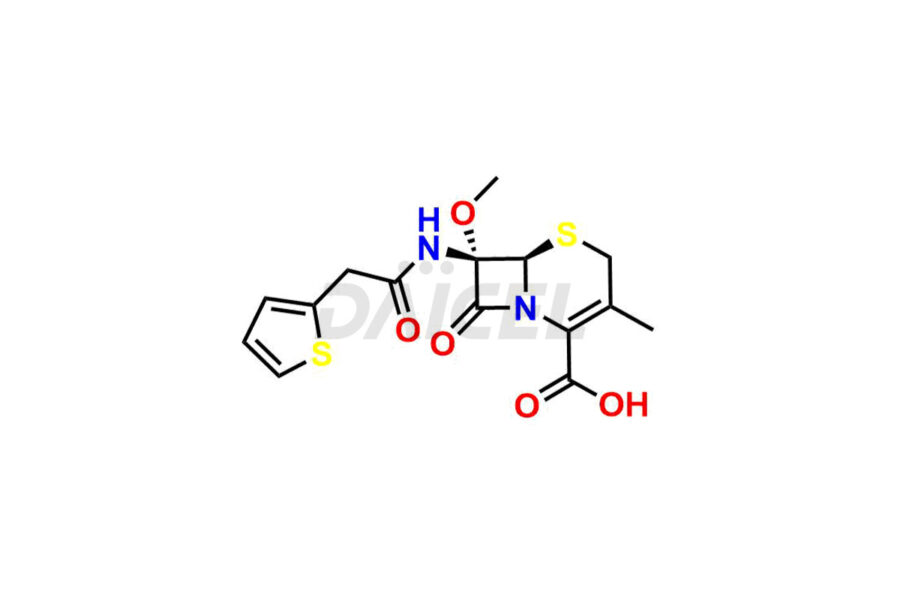
Daicel Pharma synthesizes Cefoxitin impurities of exceptional quality, such as Cefoxitin delactam amide lactone (mixture of diastereomers), Cefoxitin Delactam Lactone (Mixture of diastereomers), Cefoxitin Lactone, Descarbamoyloxy Cefoxitin, Desmethoxy Cefoxitin and Methoxy Cefoxitin 1& 2. These impurities are crucial to assess the purity, reliability, and safety of an active pharmaceutical ingredient, Cefoxitin. Besides, Daicel Pharma provides custom synthesis of Cefoxitin impurities to meet clients’ demands for delivery worldwide.
Cefoxitin [CAS: 35607-66-0] is a semi-synthetic cephamycin antibiotic with broad-spectrum antibacterial activity. It is resistant to beta-lactamase and derived from cephamycin C, which is produced by Streptomyces lactamdurans.
Cefoxitin: Use and Commercial Availability
Cefoxitin is a cephalosporin antibiotic that exhibits excellent activity against anaerobic organisms and is effective against B. fragilis. It is resistant to hydrolysis by most gram-negative β-lactamases and some gram-positive bacteria. It treats infections caused by facultative gram-negative bacilli and anaerobes. It is effective for treating pelvic inflammatory disease when combined with doxycycline. Cefoxitin is available under the tradename Mefoxin.
Cefoxitin Structure and Mechanism of Action 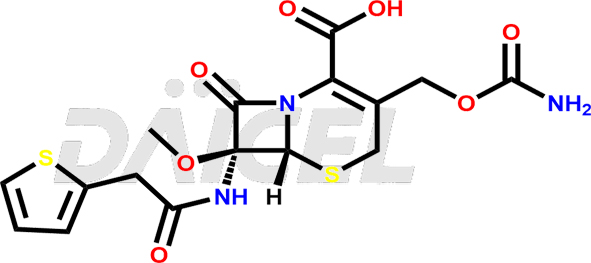
The chemical name of Cefoxitin is (6R,7S)-3-[[(Aminocarbonyl)oxy]methyl]-7-methoxy-8-oxo-7-[[2-(2-thienyl)acetyl]amino]-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid. Its chemical formula is C16H17N3O7S2, and its molecular weight is approximately 427.5 g/mol.
Cefoxitin inhibits bacterial cell wall synthesis and is active against gram-positive and gram-negative organisms.
Cefoxitin Impurities and Synthesis
Cefoxitin has impurities that appear during manufacturing1 or storage. They are related to the drug substance or the drug product. They can affect the drug’s quality, safety, and efficacy. So, it is necessary to control and monitor the Cefoxitin impurities to ensure the drug’s purity and safety. Control of Cefoxitin impurities can be through various measures such as process optimization, appropriate storage conditions, and analytical methods for impurity identification and quantification.
Daicel Pharma offers a Certificate of Analysis (CoA) for Cefoxitin impurity standards, such as Cefoxitin delactam amide lactone (mixture of diastereomers), Cefoxitin Delactam Lactone (Mixture of diastereomers), Cefoxitin Lactone, Descarbamoyloxy Cefoxitin, Desmethoxy Cefoxitin and Methoxy Cefoxitin 1& 2, generated from an analytical facility compliant with cGMP standards. The CoA includes a comprehensive characterization report comprising data from techniques like 1H NMR, 13C NMR, IR, MASS, and HPLC purity2. Furthermore, on request, we give additional data like 13C-DEPT and CHN. Daicel Pharma can synthesize unknown Cefoxitin impurities or degradation products. A complete characterization report accompanies every delivery.
References
FAQ's
References
- Christensen, Burton G.; Sletzinger, Meyer; Karady, Sandor; Cama, Lovji D., Cephalosporin antibiotics, Merck and Co., Inc., United States, US4338437A, July 6, 1982
- Wheeler, Larry A.; De Meo, Michel; Kirby, Barbara D.; Jerauld, Richard S.; Finegold, Sydney M., High-performance liquid chromatographic assay for measurement of cefoxitin in serum, Journal of Chromatography, Biomedical Applications, Volume: 183, Issue: 3, Pages: 357-62, 1980
Frequently Asked Questions
How are the impurities formed in Cefoxitin?
Impurities in Cefoxitin develop during the manufacturing process, storage, and transportation. They arise from starting materials, intermediates, or reaction by-products. Environmental factors like temperature, humidity, and light can also cause impurity formation.
How are the Cefoxitin impurities controlled?
Impurities in Cefoxitin are controlled by monitoring the manufacturing process, storing the drug under appropriate conditions, and conducting quality control tests using validated analytical methods.
What are the temperature conditions required to store Cefoxitin impurities?
Cefoxitin impurities are stored at a controlled room temperature between 2-8 °C or as indicated on the Certificate of Analysis (CoA).
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.