Carbidopa - Levodopa
General Information
Carbidopa-Levodopa Impurities and Carbidopa-Levodopa
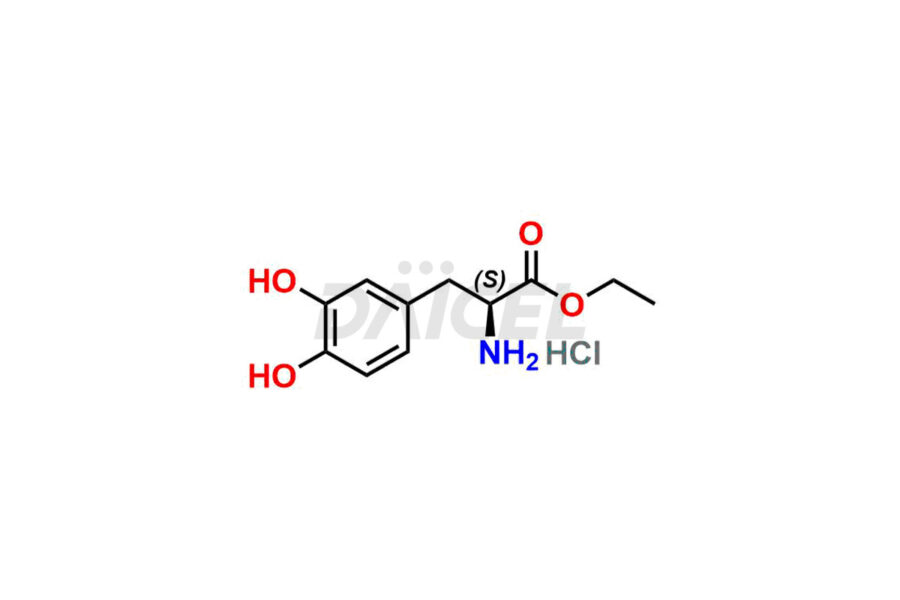
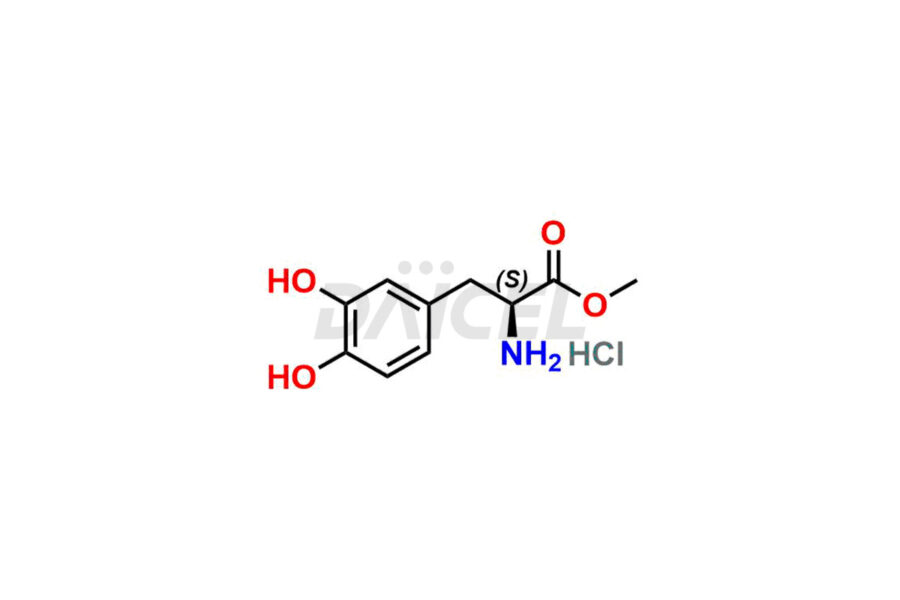
Daicel Pharma synthesizes Carbidopa-Levodopa impurities of exceptional quality, such as Etilevodopa/L-Dopa ethyl ester. HCl and Melevodopa/L-Dopa methyl ester.HCl. These impurities are crucial to assess the purity, reliability, and safety of active pharmaceutical ingredients, Carbidopa-Levodopa. Besides, Daicel Pharma provides custom synthesis of Carbidopa-Levodopa impurities to meet clients’ demands for delivery worldwide.
Carbidopa/Levodopa [CAS: 57308-51-7] is a medication that contains two active ingredients – Carbidopa and Levodopa. Carbidopa is an aromatic amino acid decarboxylation inhibitor, while Levodopa is an aromatic amino acid. Levodopa is a metabolic precursor to dopamine, a neurotransmitter deficient in Parkinson’s disease. Carbidopa-Levodopa has dopaminergic and antiparkinsonian properties and manages and treats Parkinson’s disease. Specifically, Carbidopa is used to enhance the effectiveness of Levodopa by preventing its breakdown before it can reach the brain.
Carbidopa-Levodopa: Use and Commercial Availability
Carbidopa with Levodopa treats motor symptoms associated with Parkinson’s disease, post-encephalitic parkinsonism, and parkinsonism caused by carbon monoxide or manganese intoxication. Carbidopa with Levodopa reduces the peripheral conversion of L-dopa to dopamine, decreases gastrointestinal side effects, and increases Levodopa’s bioavailability in the central nervous system. In addition, Carbidopa has potential anticancer properties through its role as a selective aryl hydrocarbon receptor modulator (SAhRM). Carbidopa-Levodopa is available under various brand names, including Carbilev, Dhivy, Duopa, Parcopa, Rytary, and Sinemet.
Carbidopa-Levodopa Structure and Mechanism of Action 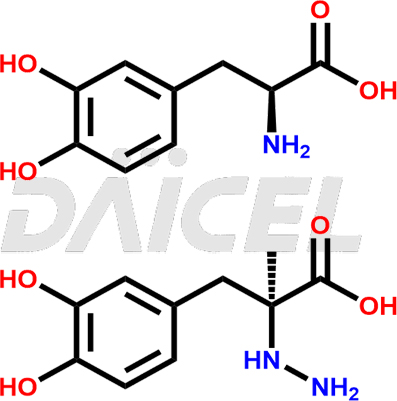
The chemical name of Carbidopa-Levodopa is 3-hydroxy-L-Tyrosine, a mixture with (αS)-α-hydrazinyl-3,4-dihydroxy-α-methylbenzenepropanoic acid. Its chemical formula is C19H25N3O8, and its molecular weight is approximately 423.4 g/mol.
Carbidopa rapidly decarboxylates to dopamine in extracerebral tissues. It inhibits the decarboxylation of peripheral Levodopa, allowing it for delivery to the brain.
Levodopa is a metabolic precursor of dopamine that can cross the blood-brain barrier and converts to dopamine in the brain.
Carbidopa-Levodopa Impurities and Synthesis
Impurities form during the synthesis or storage of Carbidopa-Levodopa combination drug products. These impurities can be related to the starting materials, by-products, or degradation products and may harm human health. It is necessary to control and minimize impurity levels to ensure the safety and efficacy of the drug product. Manufacturers use purification, filtration, and quality control testing methods to manage impurities in Carbidopa – Levodopa combination drug products.
Daicel Pharma offers a Certificate of Analysis (CoA) for Carbidopa-Levodopa impurity standards, such as Etilevodopa/L-Dopa ethyl ester. HCl and Melevodopa/L-Dopa methyl ester.HCl generated from an analytical facility compliant with cGMP standards. The CoA includes a comprehensive characterization report comprising data from techniques like 1H NMR, 13C NMR, IR, MASS, and HPLC purity1,2. Furthermore, on request, we can provide additional data like 13C-DEPT and CHN. Daicel Pharma can synthesize unknown Carbidopa – Levodopa impurities or degradation products. A complete characterization report accompanies every delivery.
References
FAQ's
References
- Mitchell, John; Coscia, Carmine J., Application of paired-ion high-pressure liquid column chromatography to the analysis of L-3,4-dihydroxyphenylalanine metabolites, Journal of Chromatography, Biomedical Applications, Volume: 145, Issue: 2, Pages: 295-301, 1978
- Damiani, Patricia C.; Moschetti, Andrea C.; Rovetto, Adrian J.; Benavente, Fernando; Olivieri, Alejandro C., Design and optimization of a chemometrics-assisted spectrophotometric method for the simultaneous determination of levodopa and carbidopa in pharmaceutical products, Analytica Chimica Acta, Volume: 543, Issue: 1-2, Pages: 192-198, 2005
Frequently Asked Questions
How are residual solvents controlled in Carbidopa-Levodopa?
Residual solvents in Carbidopa-Levodopa are controlled through strict manufacturing processes, including using appropriate solvents, purification methods, and analytical testing.
Can Carbidopa-Levodopa impurities affect the efficacy of the medication?
Impurities in Carbidopa-Levodopa can potentially affect the efficacy of the medication if they interfere with the mechanism of action of the API or if they harm patients.
Which solvent helps in the analysis of Carbidopa-Levodopa impurities?
Water is the solvent used in analyzing many impurities in Carbidopa-Levodopa.
What are the temperature conditions required to store Carbidopa-Levodopa impurities?
Carbidopa-Levodopa impurities are stored at a controlled room temperature between 2-8 °C or as indicated on the Certificate of Analysis (CoA).
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.