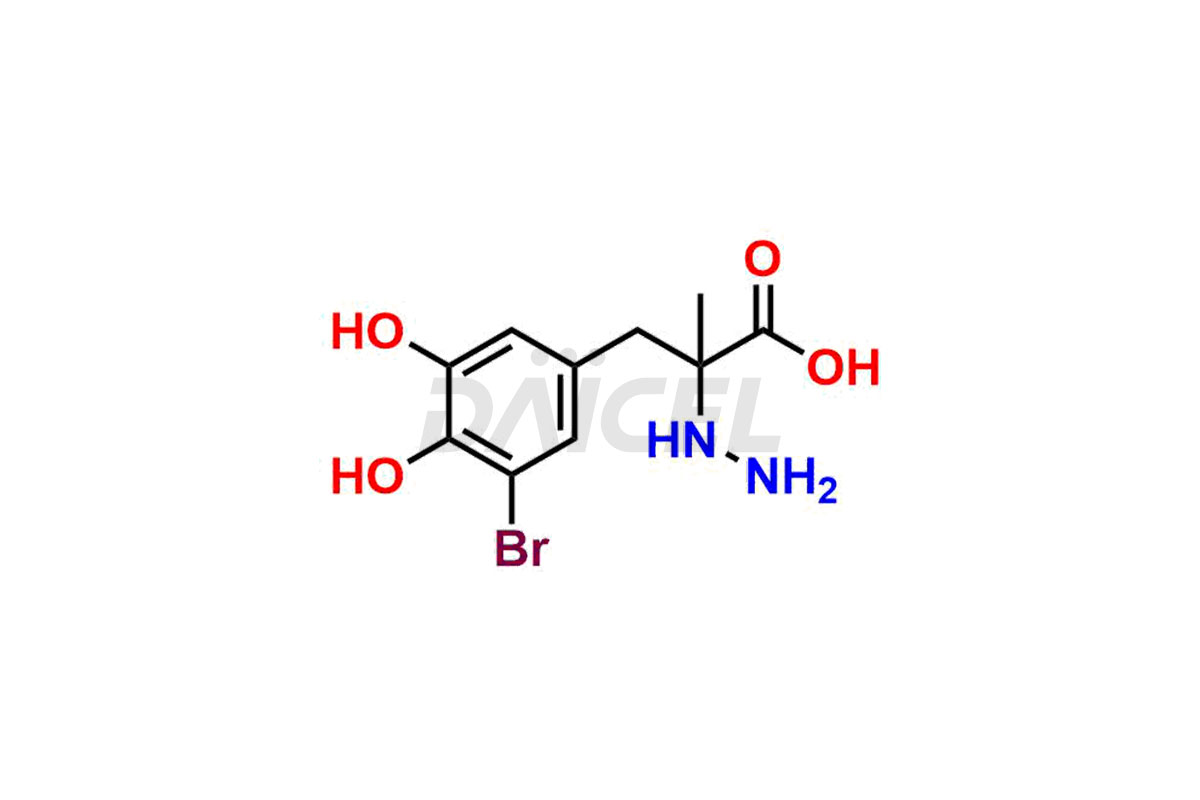
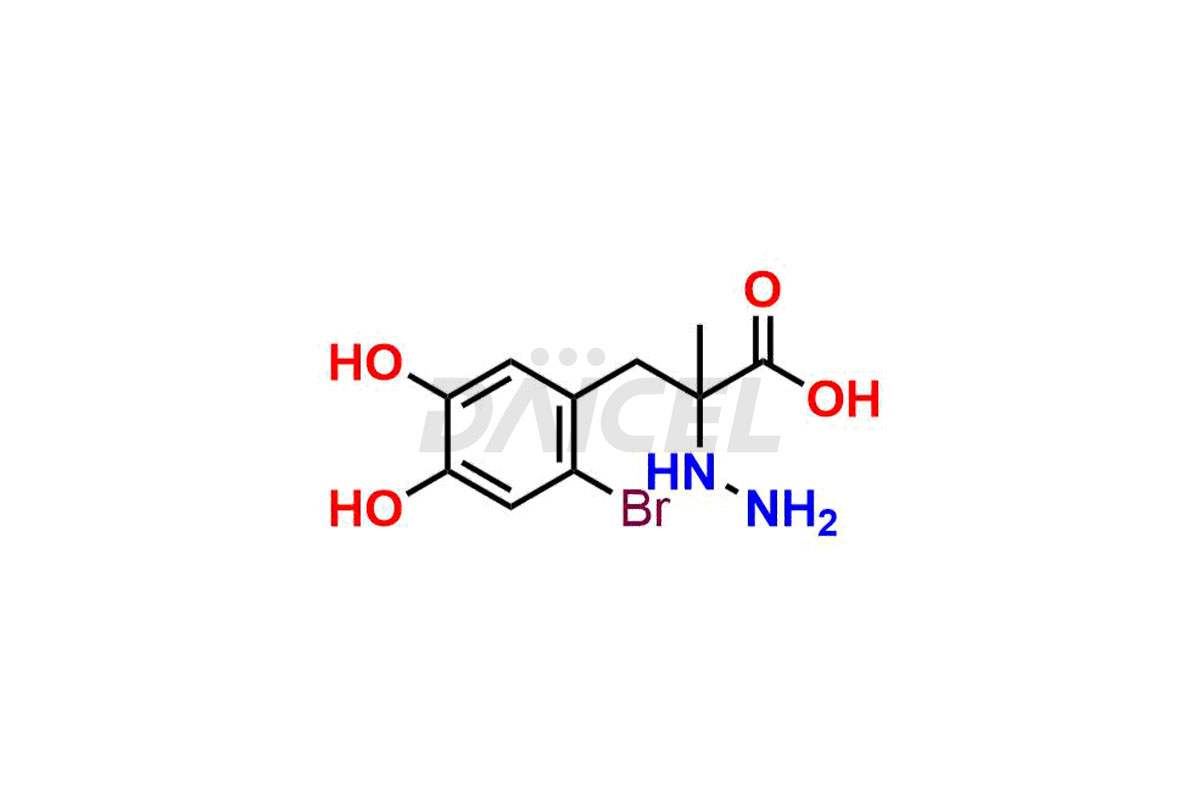
Carbidopa
General Information
Carbidopa Impurities and Carbidopa
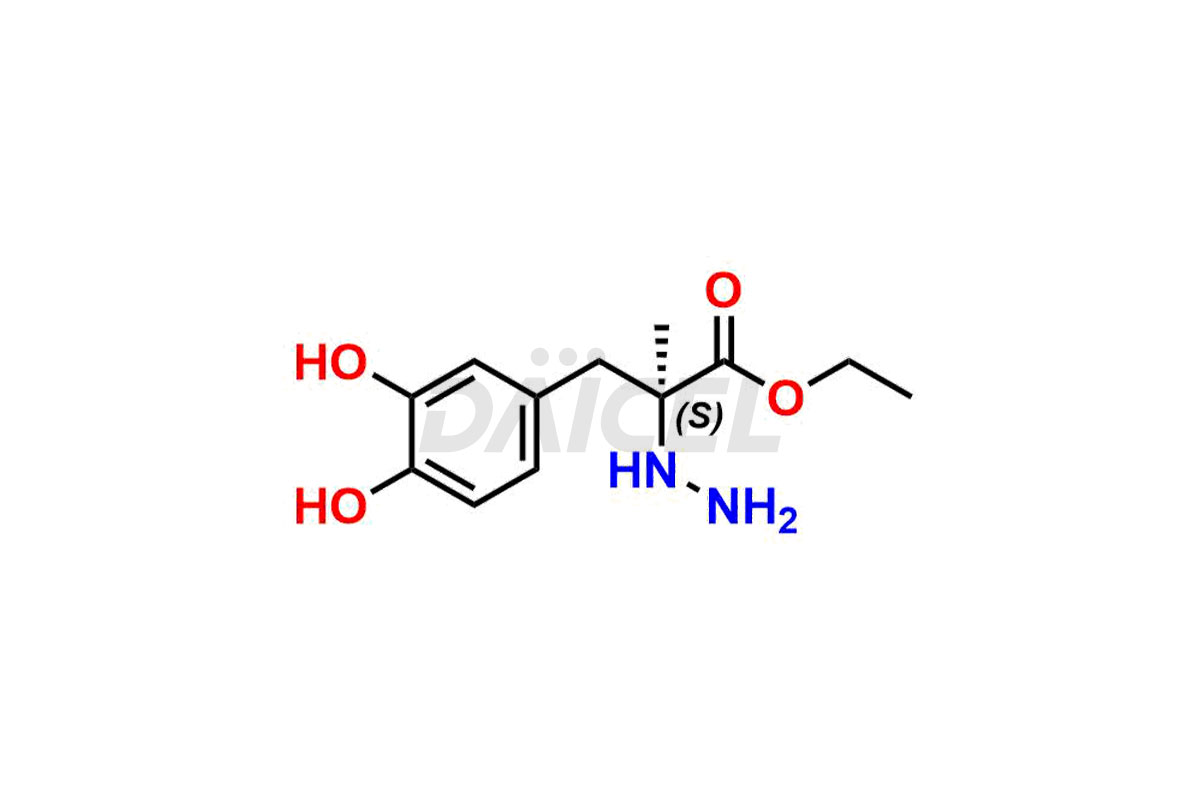
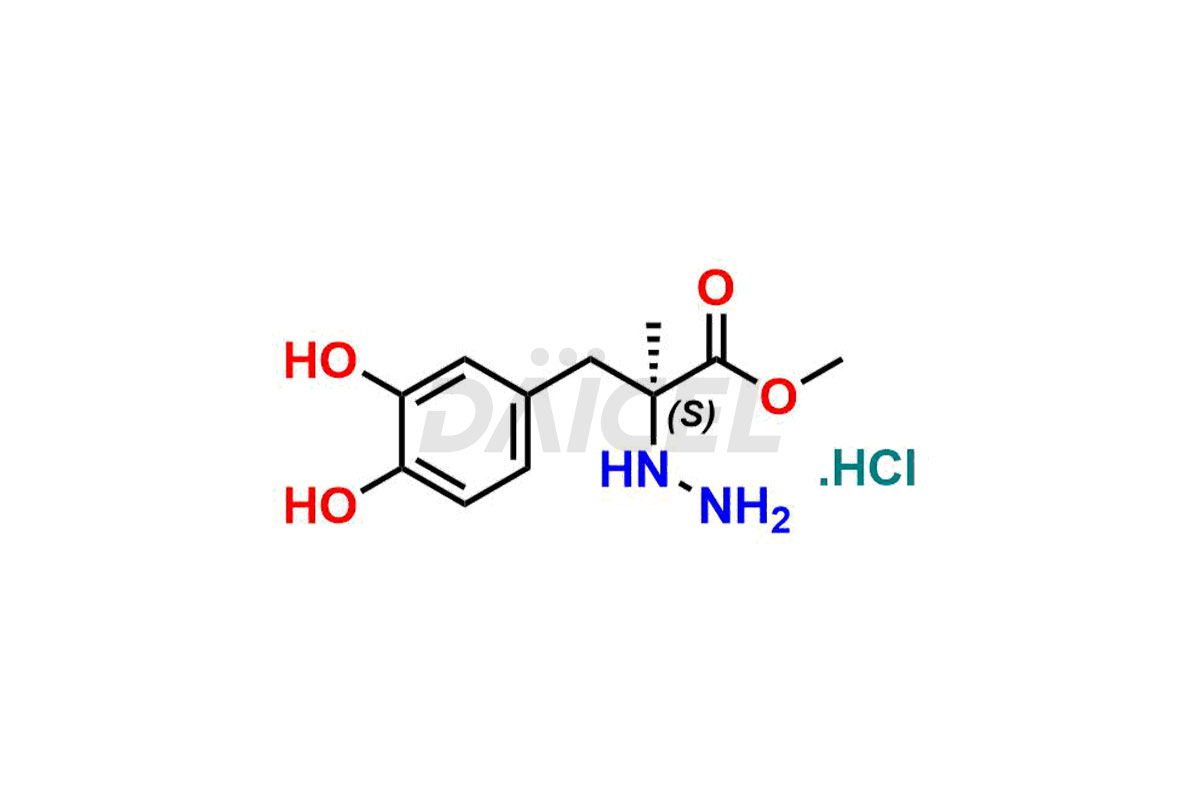
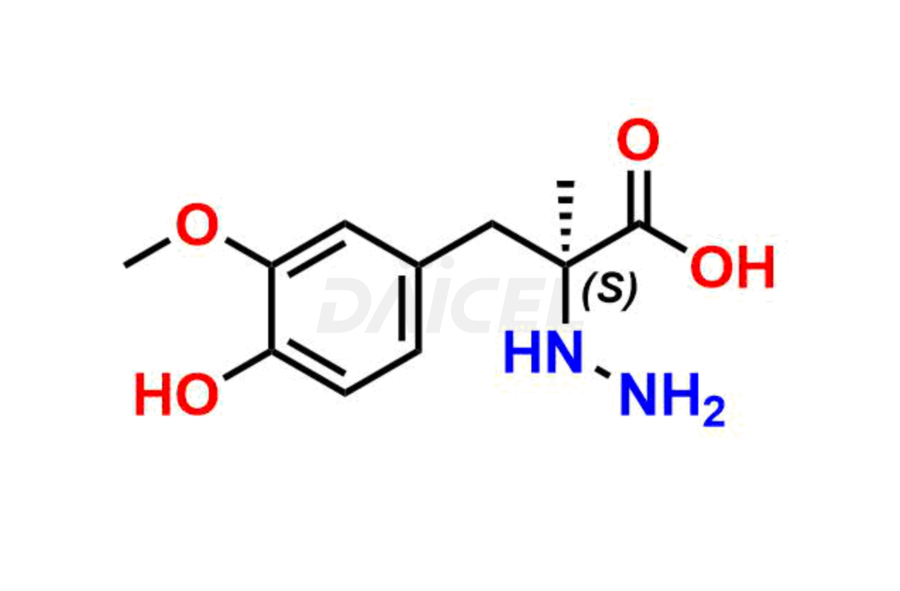
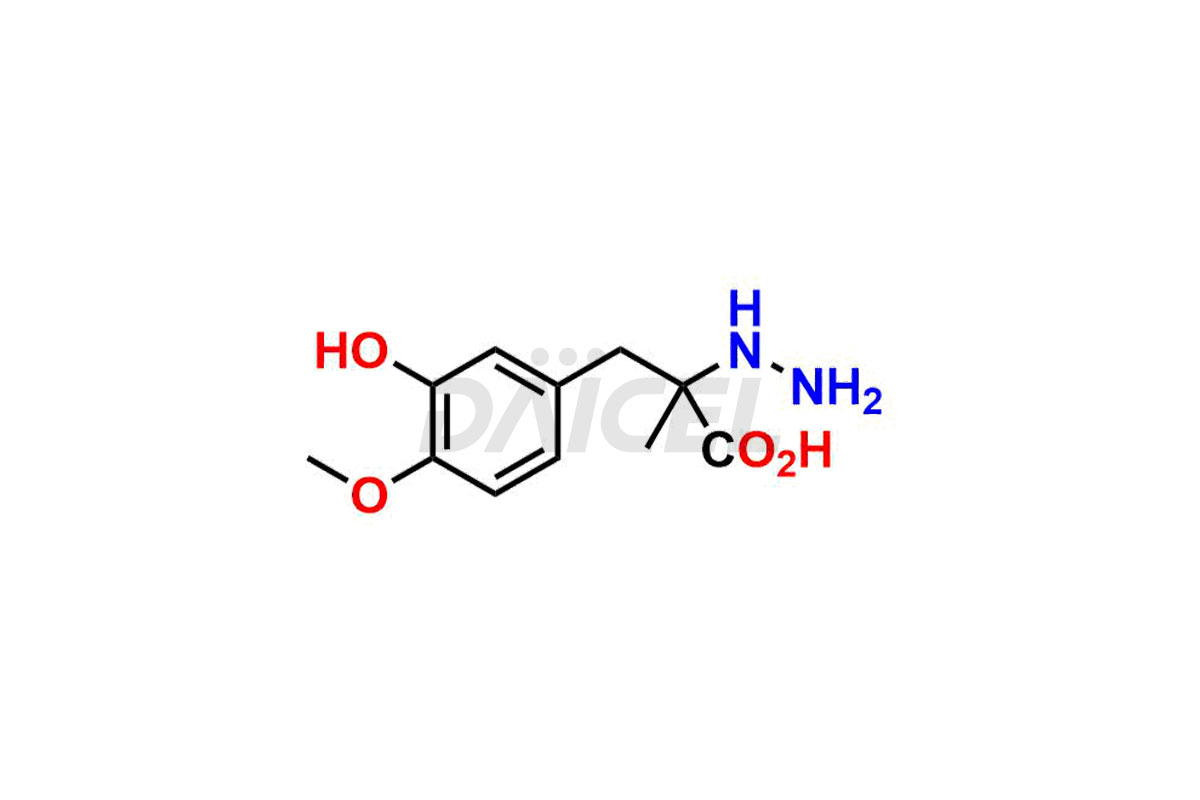
Daicel Pharma synthesizes high-quality Carbidopa impurities like (S)-Carbidopa ethyl ester, (S)-Carbidopa methyl ester, 3-Methylcinnoline-6,7 diol, Carbidopa EP Impurity C, Carbidopa EP Impurity H, Carbidopa EP Impurity I, and Carbidopa EP Impurity J, which are crucial in the analysis of the quality, stability, and biological safety of the active pharmaceutical ingredient Carbidopa. Moreover, Daicel Pharma offers custom synthesis of Carbidopa impurities and delivers them globally.
Carbidopa [CAS: 28860-95-9] is a medicine to treat Parkinson’s disease. It belongs to a class of drugs known as decarboxylase inhibitors. Carbidopa is a synthetic form of the neurotransmitter dopamine and is the levorotatory isomer of a hydrazine derivative.
Carbidopa: Use and Commercial Availability
Carbidopa is a medication used in combination with levodopa to treat motor symptoms associated with Parkinson’s disease, post-encephalitic parkinsonism, and parkinsonism symptoms caused by carbon monoxide or manganese intoxication. It is added to levodopa formulations to decrease the conversion of levodopa to dopamine outside the brain, lower gastrointestinal side effects, and improve levodopa’s effectiveness in the central nervous system. Carbidopa is available under brand names like Dhivy, Duopa, Lodosyn, Rytary, Sinemet, and Stalevo.
Carbidopa Structure and Mechanism of Action 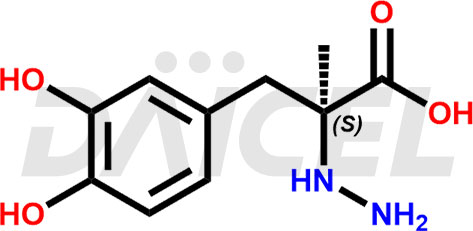
The chemical name of Carbidopa is L-α-Hydrazino-α-methyl-β-(3,4-dihydroxyphenyl)propionic acid. Its chemical formula is C10H14N2O4, and its molecular weight is approximately 226.23 g/mol.
Carbidopa inhibits peripheral decarboxylation of levodopa, the metabolic precursor of dopamine. It prevents levodopa from breaking down into dopamine in areas outside the brain, which helps decrease the harmful effects of levodopa on the body.
Carbidopa Impurities and Synthesis
During the manufacturing1,2 of Carbidopa, impurities form that can be harmful and must be carefully monitored and controlled during production. The factors that influence the formation of these impurities include reaction conditions, storage conditions, and the quality of raw materials used in the manufacturing process.
Daicel provides a Certificate of Analysis (CoA) for Carbidopa impurity standards, including (S)-Carbidopa ethyl ester, (S)-Carbidopa methyl ester, 3-Methylcinnoline-6,7 diol, Carbidopa EP Impurity C, Carbidopa EP Impurity H, Carbidopa EP Impurity I, and Carbidopa EP Impurity J. The CoA is issued from a cGMP-compliant analytical facility and contains complete characterization data, such as 1H NMR, 13C NMR, IR, MASS, and HPLC purity3. Additional characterization data, such as 13C-DEPT and CHN, can be provided upon request. Daicel can also prepare any unknown Carbidopa impurity or degradation product. We give a complete characterization report on delivery.
References
FAQ's
References
- Bayne, Gilbert M., Composition and Method Of Treating Dopamine Deficiency In Brain Tissue, Merck and Co., Inc., US3769424A, October 30, 1973
- Karady, Sandor; Ly, Manuel G.; Pines, Seemon H.; Sletzinger, Meyer, Synthesis of D- and L-α-(3,4-dihydroxybenzyl)-α-hydrazinopropionic acid via resolution, Journal of Organic Chemistry, Volume: 36, Issue: 14, Pages: 1946-8, 1971
- Vickers, S.; Stuart, E. K., Spectrofluorometric determination of carbidopa [L-(-)-α-hydrazino-3,4-dihydroxy-α-methylhydrocinnamic acid] in plasma, Journal of Pharmaceutical Sciences, Volume: 62, Issue: 9, Pages: 1550-1, 1973
Frequently Asked Questions
What methods help detect and quantify Carbidopa impurities?
The methods used to detect and quantify impurities in Carbidopa include high-performance liquid chromatography (HPLC) and liquid chromatography-mass spectrometry (LC-MS). These methods can identify and measure impurities at low levels, typically in the parts per million (ppm) range.
What is the source of impurities in Carbidopa?
The source of impurities in Carbidopa includes starting materials, reagents, catalysts, solvents, etc. The contamination also occurs during storage and transportation.
Can Carbidopa impurities vary between different batches of the drug?
The impurities in Carbidopa vary between batches of the drug, depending on factors such as the manufacturing process, the quality of the starting materials, storage, and transportation. Therefore, it is essential to test each batch of Carbidopa for impurities and ensure that it meets the regulatory requirements before its use.
What are the temperature conditions required to store Carbidopa impurities?
Carbidopa impurities are stored at a controlled room temperature between 2-8 ⁰C or as indicated on the Certificate of Analysis (CoA).
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.