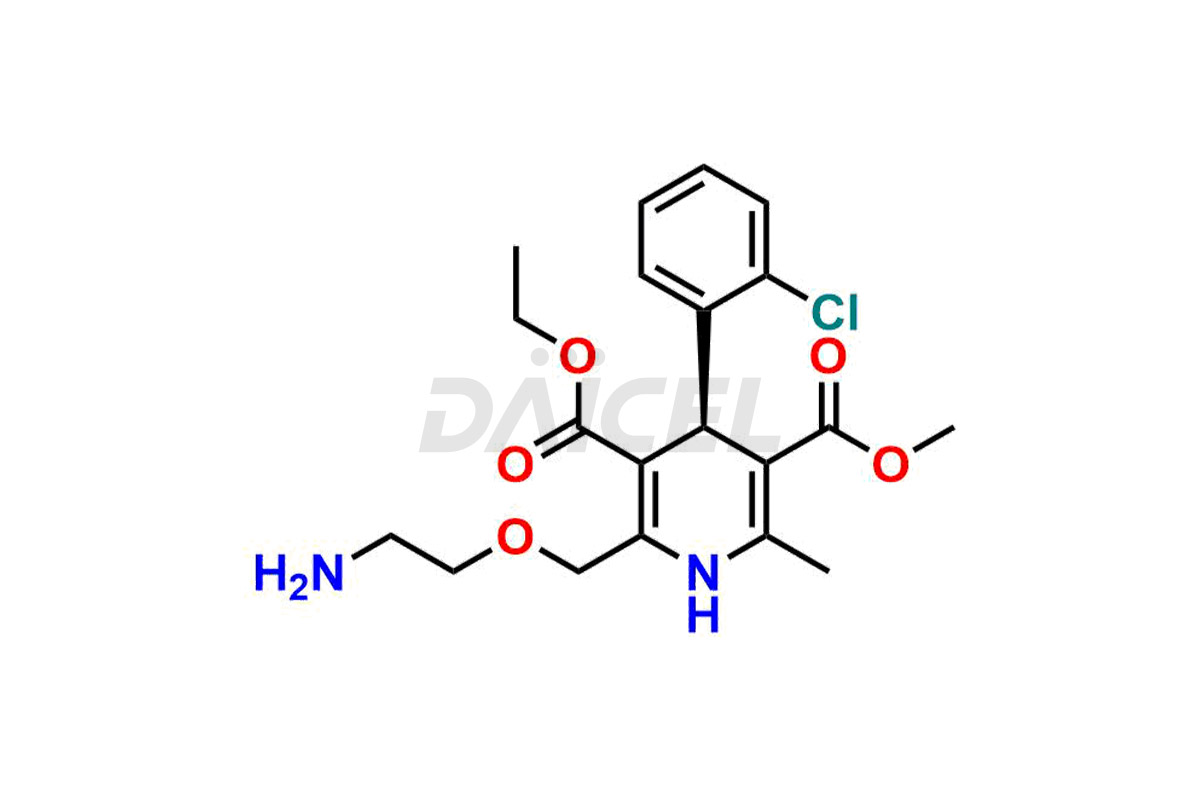
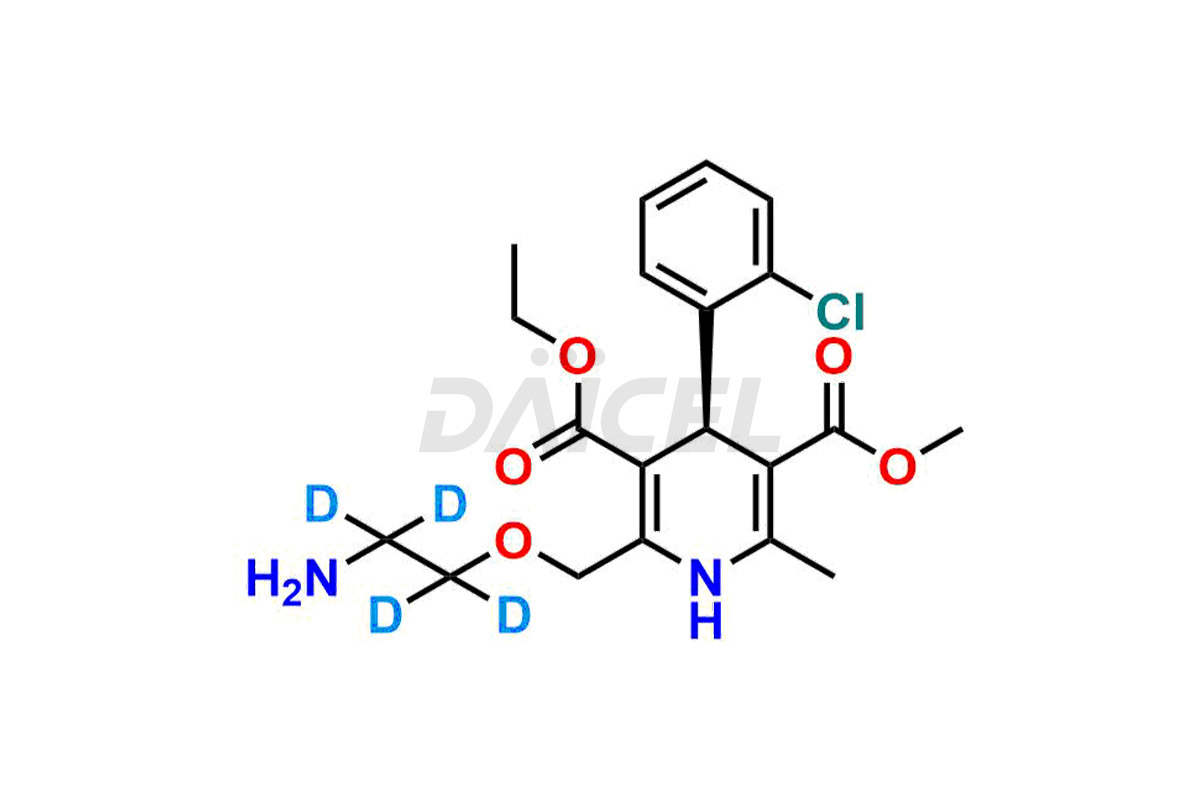
Amlodipine
References
- Campbell, Simon Fraser; Cross, Peter Edward; Stubbs, John Kendrick, Dihydropyridine Anti-Ischaemic And Antihypertensive Agents, Processes For Their Production, And Pharmaceutical Compositions Containing Them, Pfizer Ltd., United Kingdom, EP89167B1, October 15, 1986 (https://patents.google.com/patent/EP0089167B1/en)
- Pandya, K. K.; Satia, Milan; Gandhi, T. P.; Modi, I. A.; Modi, R. I.; Chakravarthy, B. K., Detection and determination of total amlodipine by high-performance thin-layer chromatography: a useful technique for pharmacokinetic studies, Journal of Chromatography B: Biomedical Sciences and Applications, Volume: 667, Issue: 2, Pages: 315-20, 1995
Frequently Asked Questions
Why is it essential to identify Amlodipine impurities?
It is essential to identify Amlodipine impurities to determine their potential impact on the safety and efficacy of the drug product.
How do Amlodipine impurities affect the pharmacokinetics of the drug?
Amlodipine impurities affect the drug’s pharmacokinetics by altering its absorption, distribution, metabolism, and elimination in the body.
Which solvent helps in the analysis of Amlodipine impurities?
Methanol or Chloroform are the solvents used for analyzing many impurities in Amlodipine.
What are the temperature conditions required to store Amlodipine impurities?
Amlodipine impurities are stored at a controlled room temperature between 2-8 °C or as indicated on the Certificate of Analysis (CoA).
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.