LOAD MORE
You're viewed 9 of 11 products
Daicel Pharma synthesizes high-quality Amlodipine impurities, including, 4-[2-(1,3-dihydro-1,3-dioxo-2H-isoindol-2-yl)ethoxy]-3-oxo-Butanoic acid ethyl ester, 2-[(2-chlorophenyl)methylene]-4-[2-(1,3-dihydro-1,3-dioxo-2H-isoindol-2-yl) ethoxy]-3-oxo-Butanoic acid ethyl ester, R-Amlodipine, S-Amlodipine, etc. These impurities are essential for evaluating the quality, stability, and safety of Amlodipine, which is an active pharmaceutical ingredient. Additionally, Daicel Pharma offers customized synthesis of Amlodipine impurities with global delivery to meet the specific needs of our customers.
Amlodipine [CAS: 88150-42-9] is a second-generation calcium channel blocker that treats hypertension and angina pectoris. It prevents the influx of extracellular calcium ions into vascular smooth muscle and myocardial cells, which causes vasodilation of the coronary and systemic arteries.
Amlodipine is an oral synthetic dihydropyridine calcium channel blocker used to treat hypertension and chronic stable angina. It blocks calcium influx into the myocardial and vascular smooth muscle cells. Amlodipine also treats vasospastic angina and Angiographically Documented Coronary artery disease (CAD). Amlodipine besylate is available either alone or in combination with other drugs. The brand names include Norliqva, Azor, Caduet, Exforge, etc.
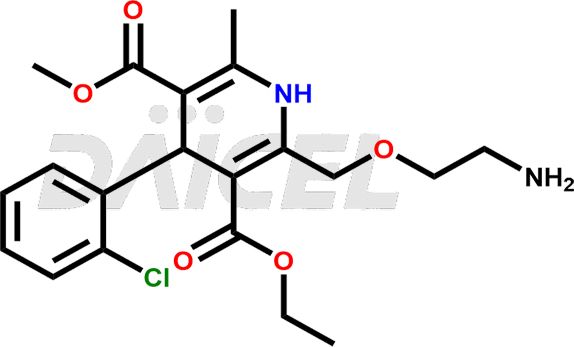
The chemical name of Amlodipine is 2-(2-Amino-ethoxymethyl)-4-(2-chloro-phenyl)-6-methyl-1,4-dihydro-pyridine-3,5-dicarboxylic acid 3-ethyl ester 5-methyl ester. Its chemical formula is C20H25ClN2O5, and its molecular weight is approximately 408.9 g/mol.
Amlodipine inhibits calcium ion influx across cell membranes affecting vascular smooth muscle cells more than cardiac muscle cells.
During manufacturing1 Amlodipine, impurities form due to degradation or reaction with other compounds that can impact the drug quality, safety, and efficacy. They may arise during the production of Amlodipine, including related substances and degradation products. These impurities can affect the stability and potency of the drug and may also harm patients. Therefore, it is necessary to control and monitor the levels of these impurities to ensure the safety and efficacy of Amlodipine as a therapeutic agent.
Daicel Pharma offers a Certificate of Analysis (CoA) for Amlodipine impurity standards, which includes 4-[2-(1,3-dihydro-1,3-dioxo-2H-isoindol-2-yl)ethoxy]-3-oxo- Butanoic acid ethyl ester, 2-[(2-chlorophenyl)methylene]-4-[2-(1,3-dihydro-1,3-dioxo-2H-isoindol-2-yl) ethoxy]-3-oxo- Butanoic acid ethyl ester, R-Amlodipine, S-Amlodipine, etc. The CoA is from a cGMP-compliant analytical facility and includes comprehensive characterization data, such as 1H NMR, 13C NMR, IR, MASS, and HPLC purity2. If requested, we give additional characterization data, such as 13C-DEPT and CHN. Daicel Pharma can create unknown Amlodipine impurities or degradation products and supply labeled compounds to evaluate the effectiveness of Amlodipine. Further, Daicel Pharma offers Amlodipine-D4, R-Amlodipine-D4 and S-Amlodipine-D4, deuterium-labeled Amlodipine compounds useful in bio-analytical research, such as BA/BE studies. Each delivery comes with a complete characterization report.
It is essential to identify Amlodipine impurities to determine their potential impact on the safety and efficacy of the drug product.
Amlodipine impurities affect the drug’s pharmacokinetics by altering its absorption, distribution, metabolism, and elimination in the body.
Methanol or Chloroform are the solvents used for analyzing many impurities in Amlodipine.
Amlodipine impurities are stored at a controlled room temperature between 2-8 °C or as indicated on the Certificate of Analysis (CoA).
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.