Importance of Cyclic Peptides and Role of Impurities
Cyclic Peptide Drugs and Their Importance
Cyclic peptides are a class of molecules that have garnered significant attention in drug development due to their unique properties, including high stability, target specificity, enhanced potency, and cell permeability. Cyclic peptides are polypeptide chains taking cyclic ring structure consisting of 5-14 amino acids with a molecular weight of about 500 to 2000 Da. The ring structure can be formed by linking one end of the peptide and the other with an amide bond, or other chemically stable bonds such as lactone, ether, thioether, disulfide, and so on.
Peptide sequence cyclization helps to bind more efficiently with their respective receptors. The cyclic structure of peptides provides a large surface area for interaction with the targeted site. Cyclic peptide drugs are impervious to enzymatic hydrolysis as they don't have free amino and carboxyl ends, thus improving their stability. Usually, cyclic peptides show better biological activity compared to their linear counterparts due to the conformational structure, therefore allowing the enhanced binding toward target molecules or the selectivity by the receptor.
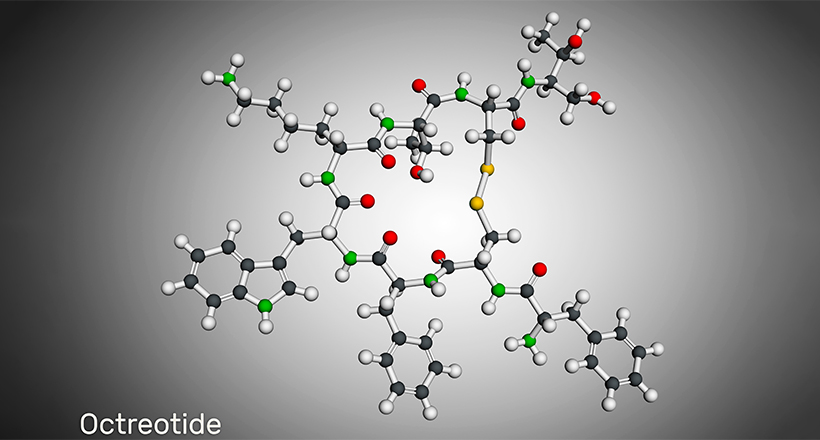
Some cyclic peptide drugs include Daptomycin, Telavancin, Dalbavancin, Lanreotide, Octreotide, Linaclotide, Plecanatide, Romidepsin, Vasopressin, Oxytocin, and Calcitonin. Many cyclic peptide drugs are from natural sources like cyclosporin A, Bactenecin, Lactocyclicin, and more. However, bio-engineered, synthetic cyclic peptide drugs are becoming common now.
How Do You Synthesize Cyclic Peptides?
The synthesis of Cyclic peptides involves Solid-Phase Peptide Synthesis (SPPS). In this method, peptide synthesis occurs linearly, immobilized on a resin bead with a disulfide linker or other chemically stable bonds such as lactone, ether, and thioether. After the linear peptide formation, possible protecting groups are cleaved, and the peptides are released by adding a base. Further, deprotonation of the N-terminal thiol group strikes the disulfide linker between the peptide and resin in intramolecular cyclization, giving the desired cyclic peptide.
Overall, synthesizing cyclic peptides requires careful control of protecting groups, coupling reagents, and reaction conditions to ensure high yields and purity.
Techniques for Synthesis of Cyclic Peptides
The preparation of cyclic peptide compounds uses various techniques: • combinatorial chemistry • de novo synthesis. • chemoselective ligation-mediated cyclization
Depending on the cyclization, the methods to synthesize cyclic peptides are head-to-tail, side-chain-to-side-chain, head-to-side-chain, and side-chain-to-tail. Cyclization of side chain amino acids occurs with disulfide bridge formation between cysteine. Further, backbone-to-backbone cyclization is by amide bond formation between N-terminal and C-terminal amino acid residues.
Moreover, there are other cyclic peptides like bicyclic/ tricyclic peptides and stapled cyclic peptides. Bicyclic peptides are effective enzyme inhibitors and stapled peptides facilitate peptide cell penetration and use cross-linkers to improve physiochemical properties.
Impurities in Cyclic Peptide Drugs
Impurities may arise from the synthesis of cyclic peptides. The API-related impurities are truncations, functional group modifications, insertion or deletion of amino acids, oxidations or reduction of functional groups, aggregates, and incomplete deprotection. Chromatographic media used in purification, and solvents also may contribute to impurity generation in cyclic peptide drugs. Degradation products occur due to changes in the drug product stored for a long time or exposure to light, temperature, water, or reaction to excipients. To ensure the safety and efficacy of cyclic peptide drugs, it is important to control and minimize impurities during the synthesis and purification processes.
General peptide impurities like N-Ac-impurity, de-amidation at Gln, Asn residues, deamidation at C-terminus, trisulfide impurity, Cyanoalanice @ Asn residue, des-Gly and endo-Gly of the terminus amino acids are common in cyclic peptides too. Degradation and other truncated peptides are also a major class of impurities in these cyclic peptides.
Apart from the above-described type of impurities, other impurities like Parallel and Anti-parallel dimers are often formed in cyclic peptides.
- Seq: Cys(1)-Tyr-Phe-Gln-Asn-Cys(6)-Pro-Arg-Gly-CONH2 (sulfur linkage between cysteines)
- Parallel dimers: 1,1’, 6,6’
- Anti-parallel dimers: 1,6’,1’,6
- Seq: H-Asn-Asp-Glu-Cys(4)-Glu-Leu-Cys(7)-Val-Asn-Val-Ala-Cys(12)-Thr-Gly-Cys(15)-Leu-OH(4-12), (7-15)-bis(disulfide)
- Parallel dimers: 4,4’, 7,7’, 12,12’, 15,15’;
- Anti-parallel dimers: 4,7’,4’,7, 12,15’, 12’,15; or 4,12’,4’,12,7,15’,7’,15 or 4,15’,4’,15, 7,12’,7’,12
- Seq: H-Cys(1)-Cys(2)-Glu-Tyr-Cys(5)-Cys(6)-Asn-Pro-Ala-Cys(10)-Thr-Gly-Cys(13)-Tyr-OH (3 disulfide bridges between 1-6, 2-10 and 5-13 of cysteines)