Ziprasidone
General Information
Ziprasidone Impurities and Ziprasidone
Daicel Pharma is a trusted provider of quality Ziprasidone impurity standards, including Ziprasidone Related Compound B and Ziprasidone Related Compound C. These impurities are crucial in meticulously evaluating the quality, stability, and safety of the active pharmaceutical ingredient, Ziprasidone. Furthermore, Daicel Pharma customizes Ziprasidone impurities to meet client specifications. With global shipping capabilities, these impurities can be conveniently delivered to customers worldwide, providing unparalleled convenience.
Ziprasidone [CAS: 146939-27-7] is an atypical antipsychotic for treating schizophrenia and bipolar disorder.
Ziprasidone: Use and Commercial Availability
Ziprasidone treats schizophrenia and acts as monotherapy for managing acute manic or mixed episodes associated with bipolar I disorder. It combines with lithium or valproate for the maintenance treatment of bipolar I disorder.
Ziprasidone is available under the brand Geodon, which contains the active ingredient Ziprasidone.
Ziprasidone Structure and Mechanism of Action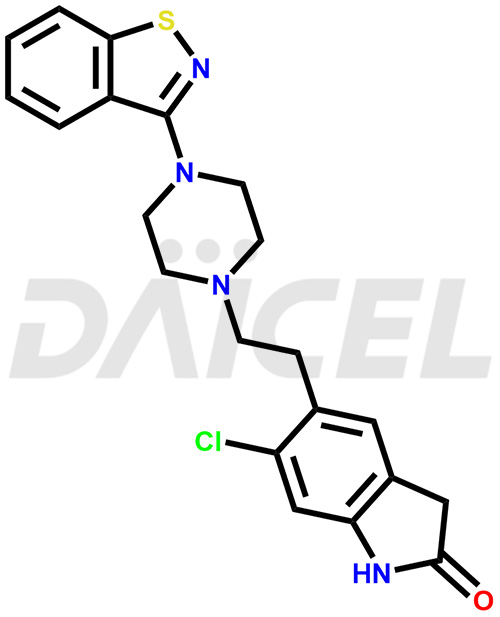
The chemical name of Ziprasidone is 5-[2-[4-(1,2-Benzisothiazol-3-yl)-1-piperazinyl]ethyl]-6-chloro-1,3-dihydro-2H-indol-2-one. Its chemical formula is C21H21ClN4OS, and its molecular weight is approximately 412.9 g/mol.
Ziprasidone binds to dopamine-2 and serotonin-2A receptors contributing to its antidepressant activity.
Ziprasidone Impurities and Synthesis
Ziprasidone impurities can arise during synthesis1 due to the storage or use of specific raw materials and intermediates in manufacturing. These impurities encompass related compounds, degradation products, and process impurities. Stringent quality control measures and analytical methods are crucial to ensure the purity and safety of Ziprasidone for patient use.
Daicel Pharma provides a comprehensive Certificate of Analysis (CoA) for Ziprasidone impurity standards, such as Ziprasidone Related Compound B and Ziprasidone Related Compound C. The CoA includes detailed characterization data such as 1H NMR, 13C NMR, IR, MASS, and HPLC purity2. Additionally, upon delivery, we give 13C-DEPT. Daicel possesses the technology and expertise to synthesize any unknown Ziprasidone impurity or degradation product.
References
FAQ's
References
- Bowles, Paul, Process for preparing aryl piperazinyl-heterocyclic compounds, Pfizer Inc, US5206366A, April 27, 1993
- Suckow, Raymond F.; Fein, Mira; Correll, Christoph U.; Cooper, Thomas B., Determination of plasma ziprasidone using liquid chromatography with fluorescence detection, Journal of Chromatography B: Analytical Technologies in the Biomedical and Life Sciences Volume: 799, Issue: 2, Pages: 201-208, 2004
Frequently Asked Questions
Can Ziprasidone impurities affect the drug's bioavailability?
Impurities in Ziprasidone can affect the drug's bioavailability and its therapeutic effect.
What are the sources of Ziprasidone impurities?
Impurities in Ziprasidone can come from the starting materials, intermediates, degradation products, or residual solvents.
How should Ziprasidone impurities be stored in terms of temperature?
The recommendation is to store Ziprasidone impurities at room temperature, within 2-8 °C.
Which solvent helps in the analysis of Ziprasidone impurities?
DMSO is the solvent used in analyzing impurities in Ziprasidone.
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.