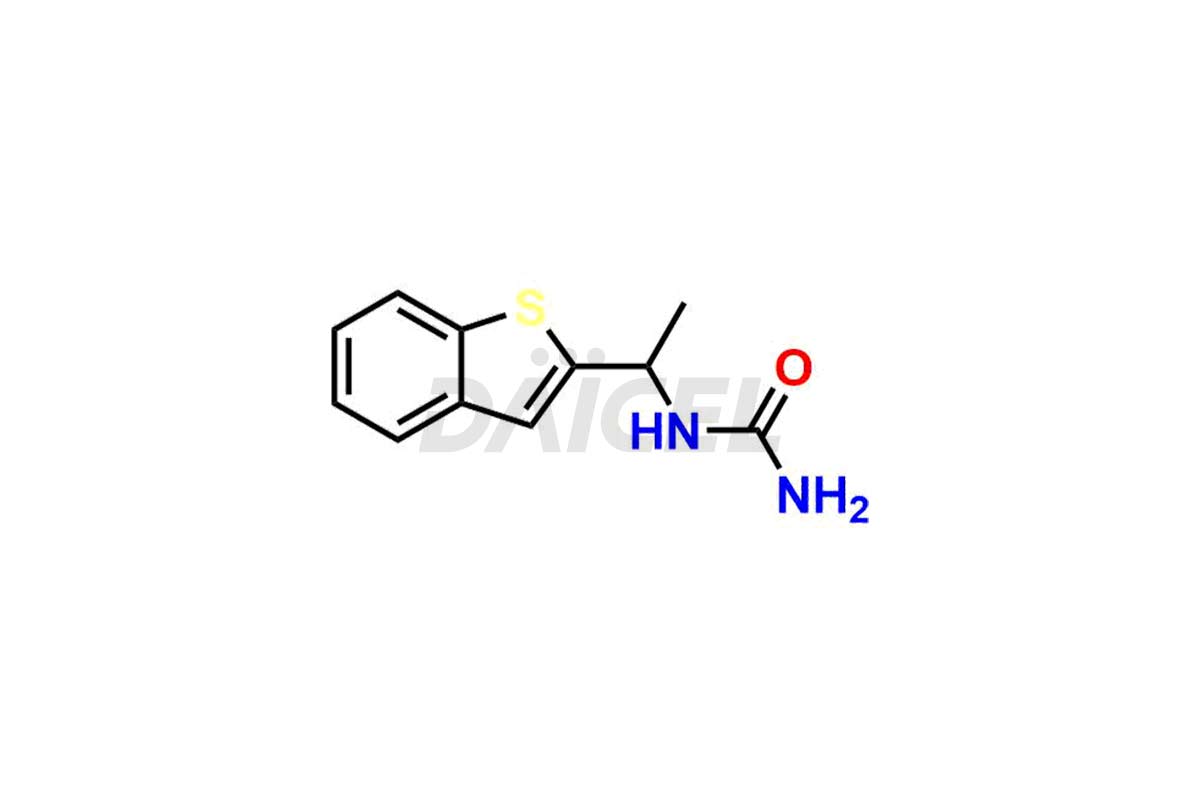
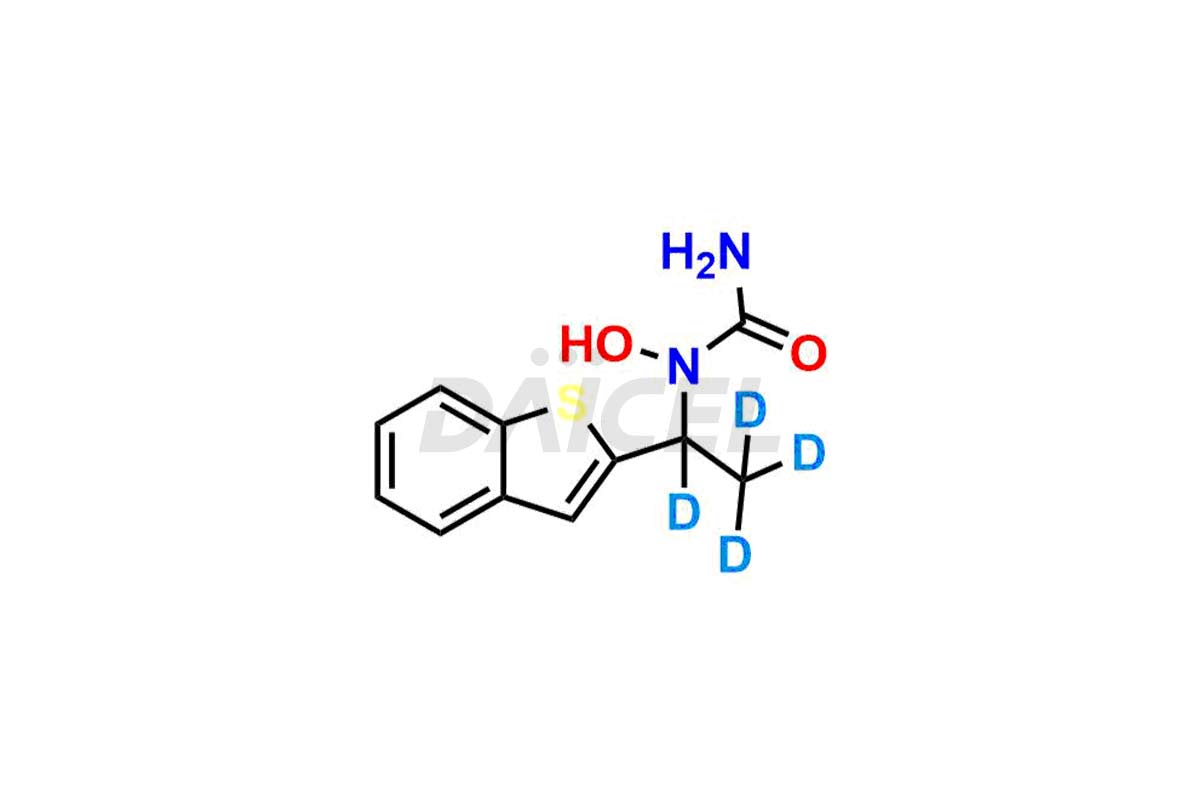
Zileuton
General Information
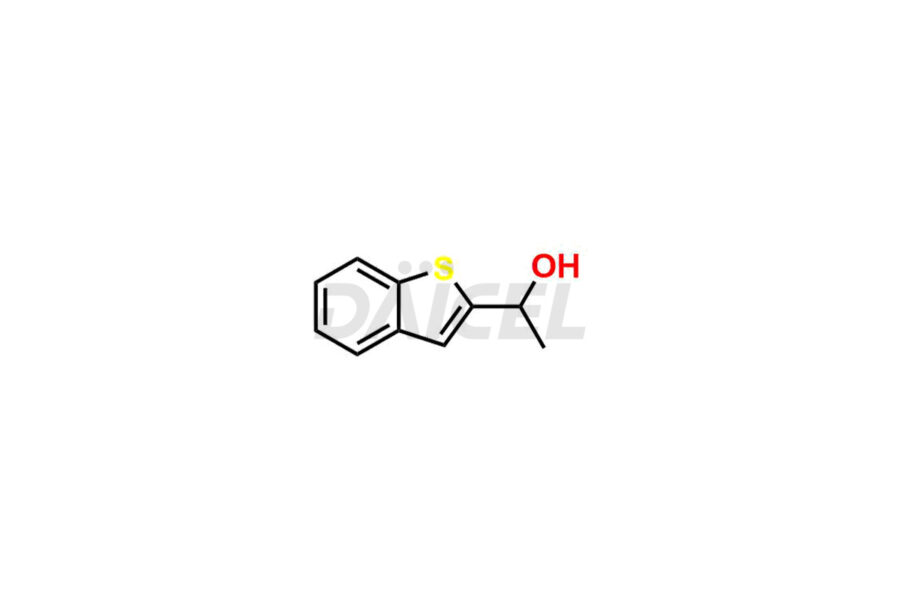
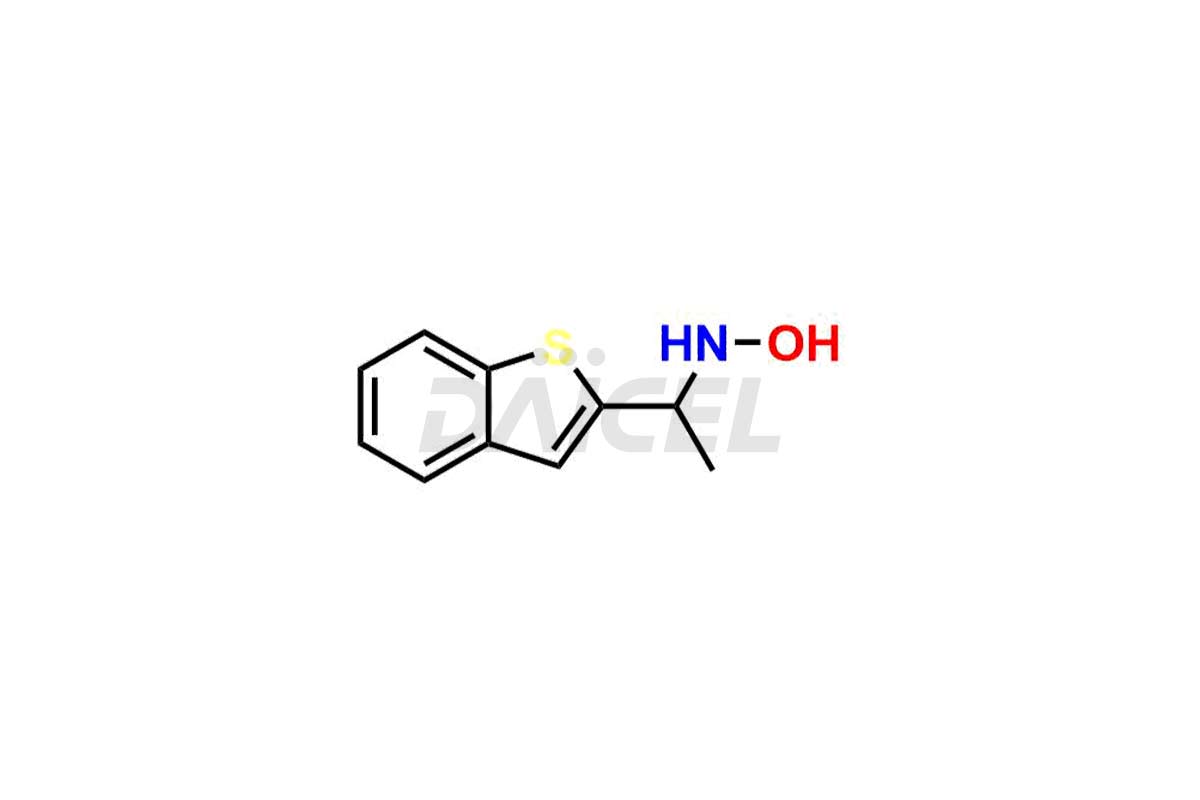
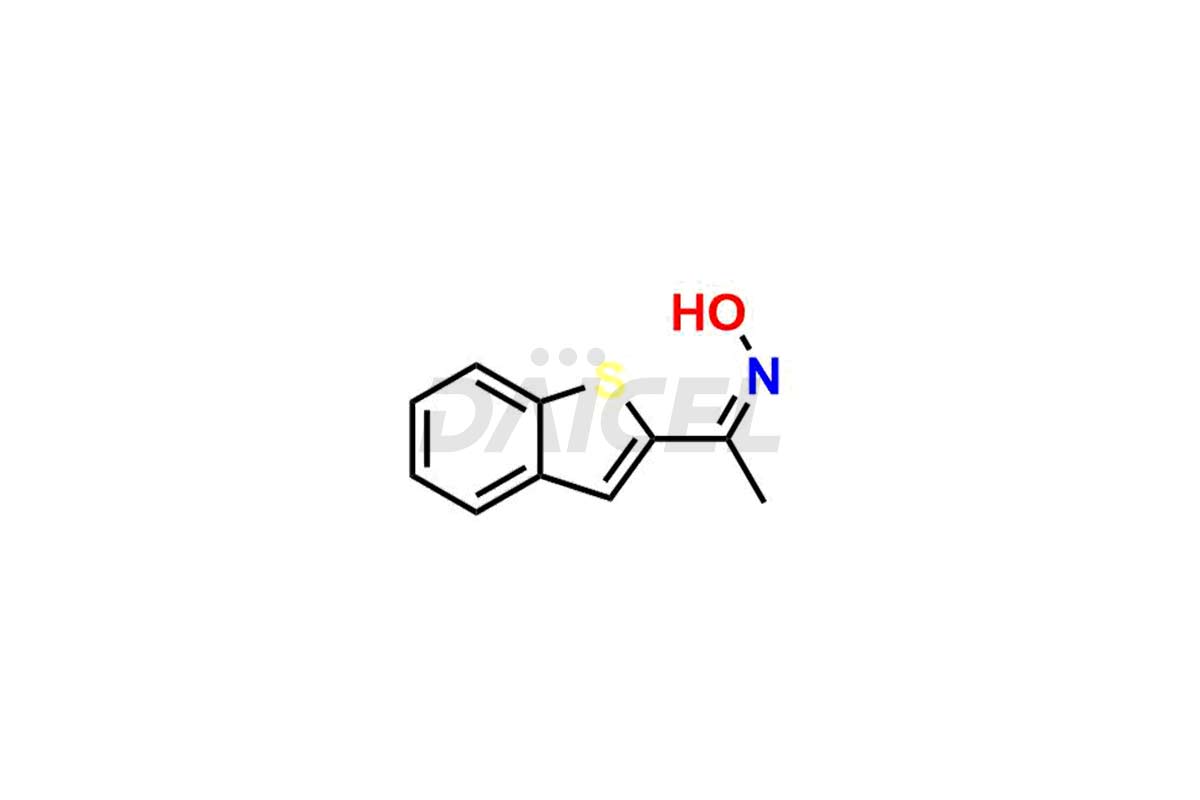
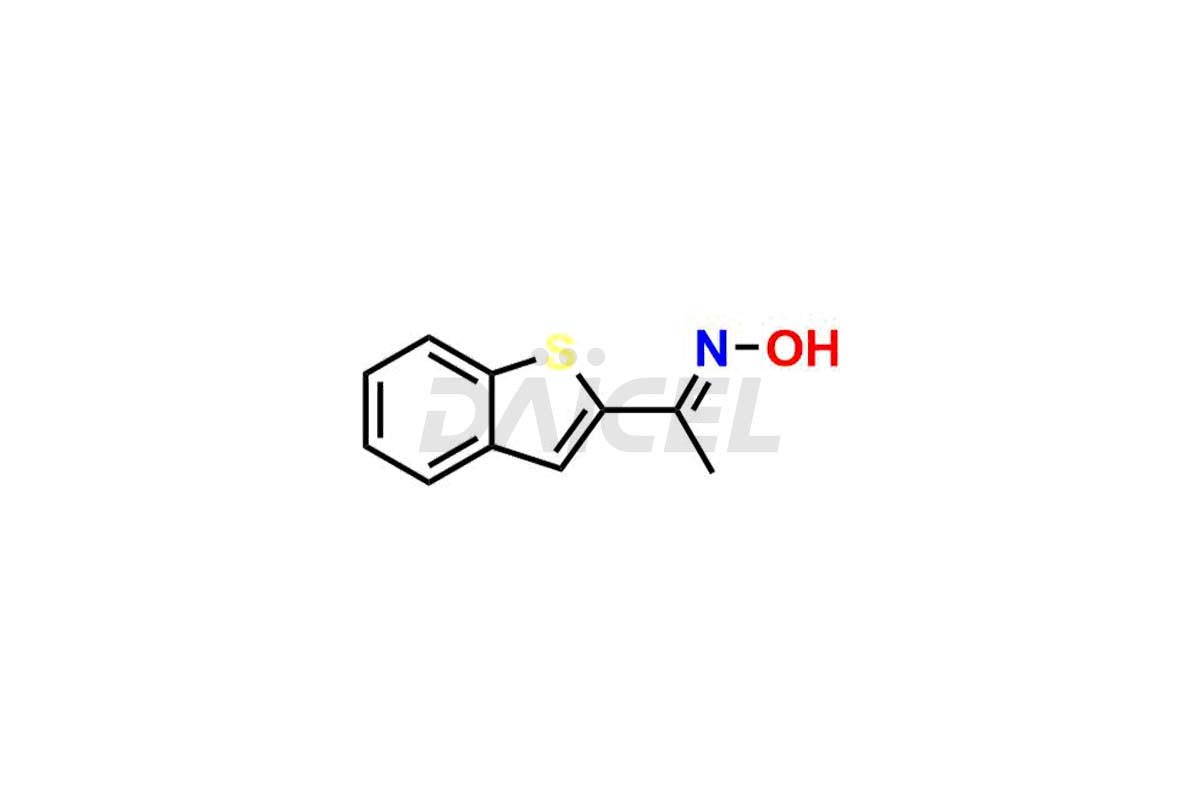
Zileuton Impurities and Zileuton
Daicel Pharma is a trusted provider of quality Zileuton impurity standards, including 2-ABT Impurity, Hydroxy benzo thiophene, Zileuton impurity-A, Zileuton impurity-C, Zileuton impurity-B, and Zileuton impurity-D. These impurities are crucial in meticulously evaluating the quality, stability, and safety of the active pharmaceutical ingredient, Zileuton. Furthermore, Daicel Pharma customizes Zileuton impurities to meet client specifications. With global shipping capabilities, these impurities can be conveniently delivered to customers worldwide, providing unparalleled convenience.
Zileuton [CAS: 111406-87-2] is a 5-lipoxygenase inhibitor to prevent acute asthma attacks. It is for long-term asthma prevention in patients who require continuous treatment to control their asthma symptoms.
Zileuton: Use and Commercial Availability
Zileuton primarily treats asthma in adults and children aged 12 and above. It helps manage chronic asthma symptoms and prevent asthma attacks. Additionally, Zileuton is effective in preventing exercise-induced bronchoconstriction (EIB). It works by reducing inflammation and improving airflow in the airways. Zileuton is available under the brand name Zyflo and Zyflo-CR.
Zileuton Structure and Mechanism of Action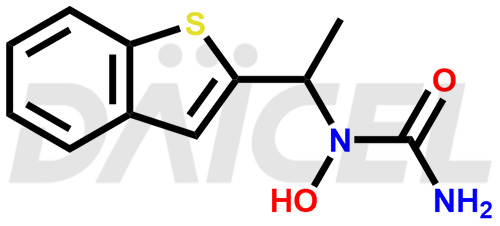
The chemical name of Zileuton is N-(1-Benzo[b]thien-2-ylethyl)-N-hydroxyurea. Its chemical formula is C11H12N2O2S, and its molecular weight is approximately 236.29 g/mol.
Zileuton blocks 5-lipoxygenase and prevents leukotriene formation.
Zileuton Impurities and Synthesis
Zileuton impurities can arise during synthesis1 due to the storage or use of specific raw materials and intermediates in manufacturing. These impurities encompass related compounds, degradation products, and process impurities. Stringent quality control measures and analytical methods are crucial to ensure the purity and safety of Zileuton for patient use.
Daicel Pharma provides a comprehensive Certificate of Analysis (CoA) for Zileuton impurity standards, such as 2-ABT Impurity, Hydroxy benzo thiophene, Zileuton impurity-A, Zileuton impurity-C, Zileuton impurity-B, and Zileuton impurity-D. The CoA includes detailed characterization data such as 1H NMR, 13C NMR, IR, MASS, and HPLC purity2. Additionally, upon delivery, we give 13C-DEPT. Daicel possesses the technology and expertise to synthesize any unknown Zileuton impurity or degradation product. We also offer labeled compounds to quantify the efficacy of generic Zileuton. For bioanalytical research and BA/BE studies, Daicel offers Zileuton Impurity-D4, a deuterium-labeled Zileuton standard.
References
FAQ's
References
- Summers, James B., Jr.; Gunn, Bruce P.; Brooks, Dee W., Indole, benzofuran, benzothiophene containing lipoxygenase inhibiting compounds, Abbott Laboratories, United States, EP0279263A2, August 24, 1988
- Pian, Phillip; Labovitz, Edward; Hoffman, Keith; Clavijo, Claudia F.; Rzasa Lynn, Rachael; Galinkin, Jeffrey L.; Vinks, Alexander A.; Malik, Punam; Christians, Uwe, Quantification of the 5-lipoxygenase inhibitor zileuton in human plasma using high performance liquid chromatography-tandem mass spectrometry, Journal of Chromatography B: Analytical Technologies in the Biomedical and Life Sciences, Volume: 937, Pages: 79-83, 2013
Frequently Asked Questions
Are there regulatory limits for Zileuton impurities?
Regulatory authorities establish limits for impurities in pharmaceutical products, including Zileuton. These limits define the acceptable impurity levels considered safe for human consumption.
How are Zileuton impurities evaluated?
Zileuton impurities are evaluated through comprehensive testing with analytical methods and techniques such as Ultra-High-Pressure Liquid Chromatography.
How should Zileuton impurities be stored in terms of temperature?
The recommendation is to store Zileuton impurities at room temperature, within 2-8 °C.
Which solvent helps in the analysis of Zileuton impurities?
Methanol is the solvent used in analyzing many impurities in Zileuton.
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.