Vilazodone
General Information
Vilazodone Impurities and Vilazodone
Daicel Pharma is a trusted provider of quality Vilazodone impurity standards, including Vilazodone Metabolite 11 and Vilazodone metabolite-10. These impurities are crucial in meticulously evaluating the quality, stability, and safety of the active pharmaceutical ingredient, Vilazodone. Furthermore, Daicel Pharma customizes Vilazodone impurities guaranteeing to meet individual client specifications. With global shipping capabilities, these impurities can be conveniently delivered to customers worldwide, offering unparalleled convenience.
Vilazodone [CAS: 163521-12-8] is utilized in treating major depressive disorders and belongs to a class of medications known as selective serotonin reuptake inhibitors (SSRIs) and partial serotonin receptor agonists.
Vilazodone: Use and Commercial Availability
Vilazodone is a medication that treats major depressive disorders. It belongs to a class of drugs known as selective serotonin reuptake inhibitors (SSRIs) and partial serotonin receptor agonists. By inhibiting the reuptake of serotonin and acting as a partial agonist on serotonin receptors, Vilazodone helps to regulate and balance serotonin levels in the brain. Vilazodone is available under the brand name Viibryd.
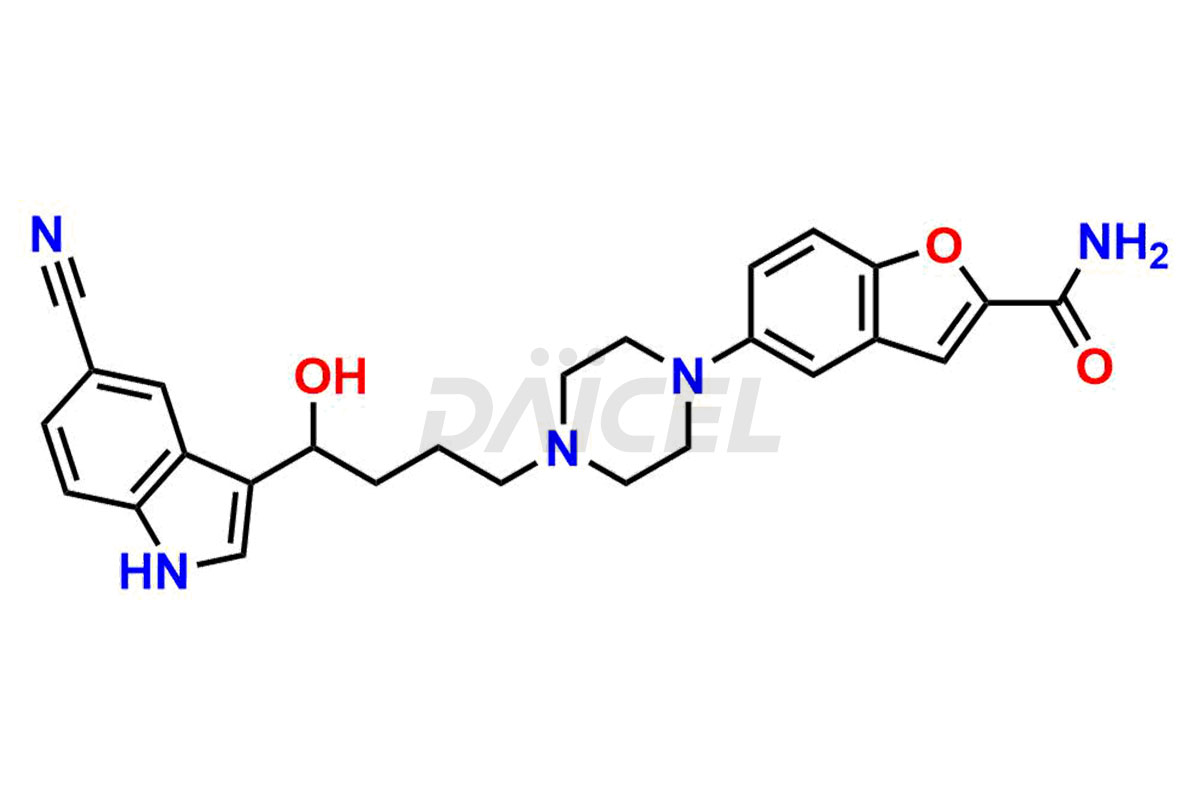
Vilazodone Structure and Mechanism of Action
The chemical name of Vilazodone is 5-[4-[4-(5-Cyano-1H-indol-3-yl)butyl]-1-piperazinyl]-2-benzofurancarboxamide. Its chemical formula is C26H27N5O2, and its molecular weight is approximately 441.5 g/mol.
Vilazodone selectively inhibits serotonin reuptake resulting in serotonergic activity in the Central nervous system.
Vilazodone Impurities and Synthesis
Vilazodone impurities can arise during synthesis1 due to the storage or use of specific raw materials and intermediates in manufacturing. These impurities encompass related compounds, degradation products, and process impurities. Stringent quality control measures and analytical methods are crucial to ensure the purity and safety of Vilazodone for patient use.
Daicel Pharma provides a comprehensive Certificate of Analysis (CoA) for Vilazodone impurity standards, such as Vilazodone Metabolite 11 and Vilazodone metabolite-10. The CoA includes detailed characterization data such as 1H NMR, 13C NMR, IR, MASS, and HPLC purity2. Additionally, upon delivery, we give 13C-DEPT. Daicel Pharma possesses the technology and expertise to synthesize any unknown Vilazodone impurity or degradation product.
References
FAQ's
References
- Boettcher, Henning; Seyfried, Christoph; Bartoszyk, Gerd; Greiner, Hartmut, Piperdine and piperazine derivatives which affect the C.N.S., Merck Patent G.m.b.H., Germany, EP648767B1, May 28, 1997
- El-Bagary, Ramzia; Hashem, Hanaa; Fouad, Marwa; Tarek, Sally, UPLC-MS-MS method for the determination of vilazodone in human plasma: application to a pharmacokinetic study, Journal of Chromatographic Science, Volume: 54, Issue: 8, Pages: 1365-1372, 2016
Frequently Asked Questions
Why is it essential to control impurities in Vilazodone?
Controlling impurities in Vilazodone is crucial to ensure the drug’s quality, safety, and efficacy. They can affect Vilazodone stability, pharmacological activity, and toxicity and interfere with the analytical methods used for its detection and quantification.
What is the acceptable limit for Vilazodone impurities?
Regulatory agencies like the US FDA, EMA, and ICH establish acceptable limits for impurities in Vilazodone. The limits may vary depending on the impurities ensuring that Vilazodone meets stringent standards for its quality and suitability for patient use.
How are Vilazodone impurities controlled during the manufacturing process of the drug?
Impurities in Vilazodone are controlled during the synthetic process by implementing good manufacturing practices (GMP) and using appropriate analytical methods for impurity identification and quantification.
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.