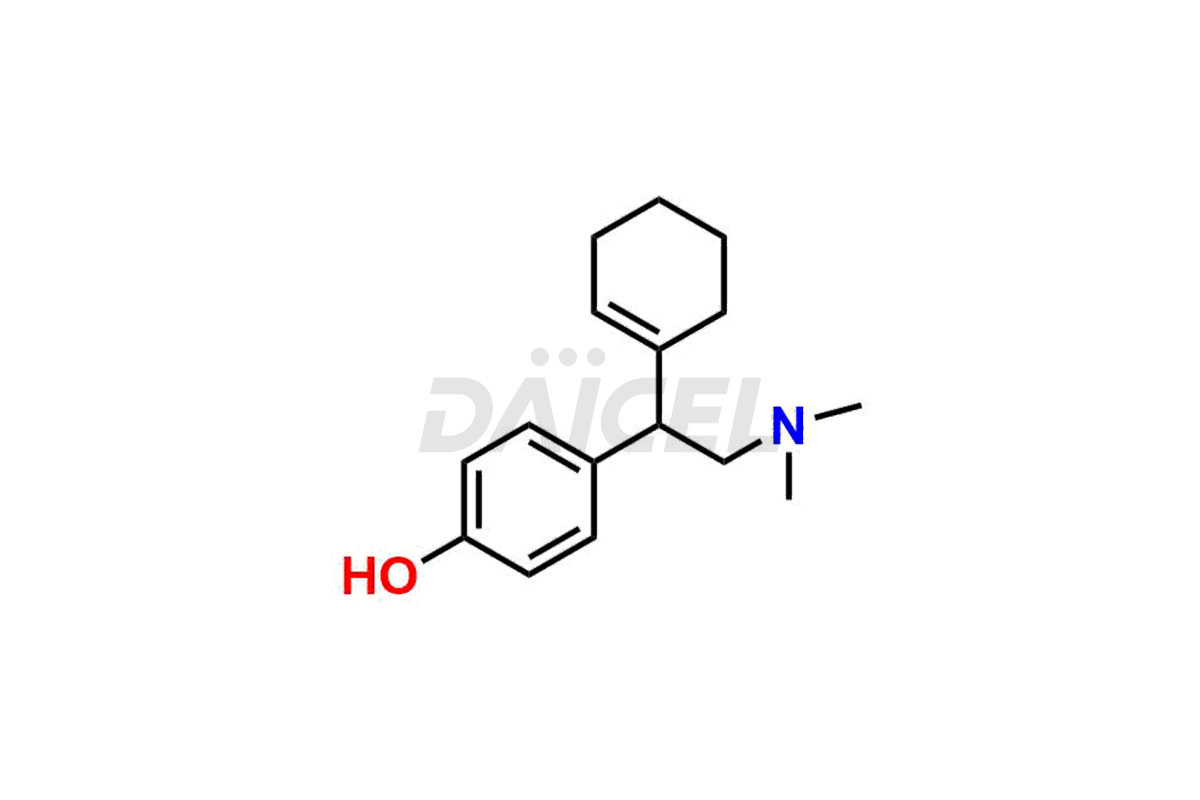
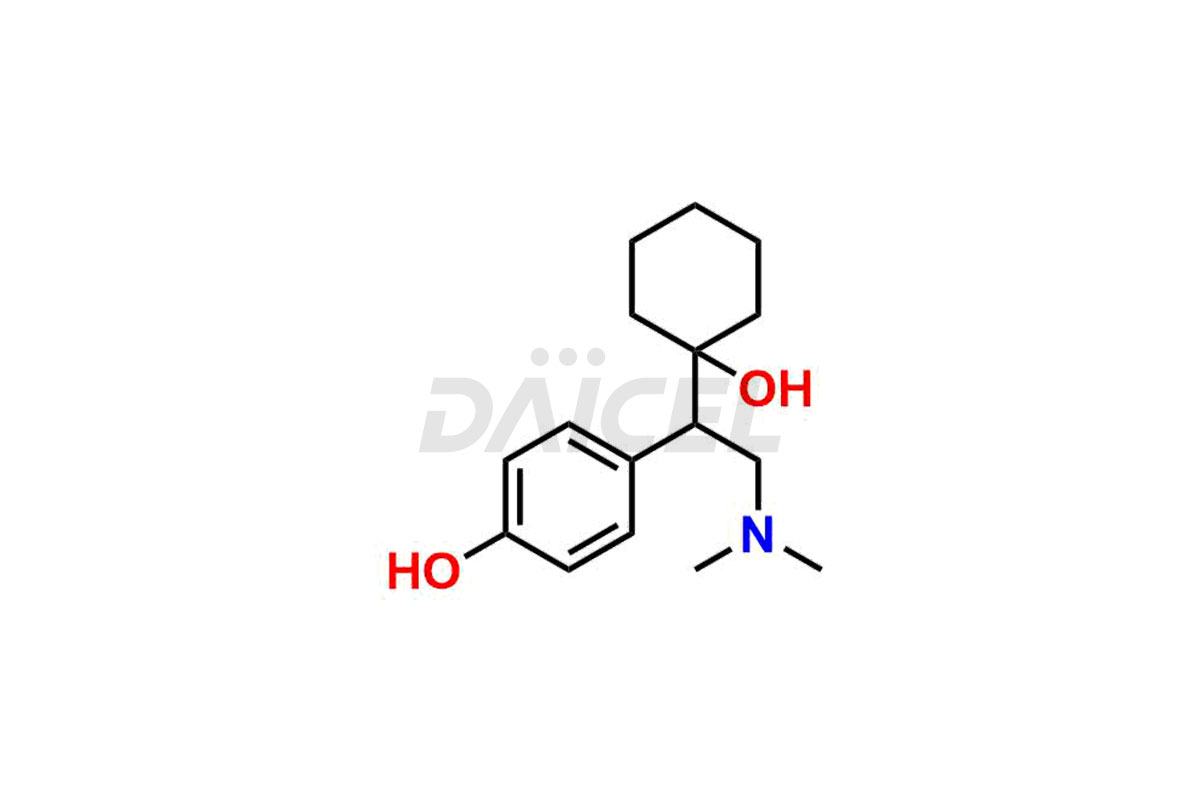
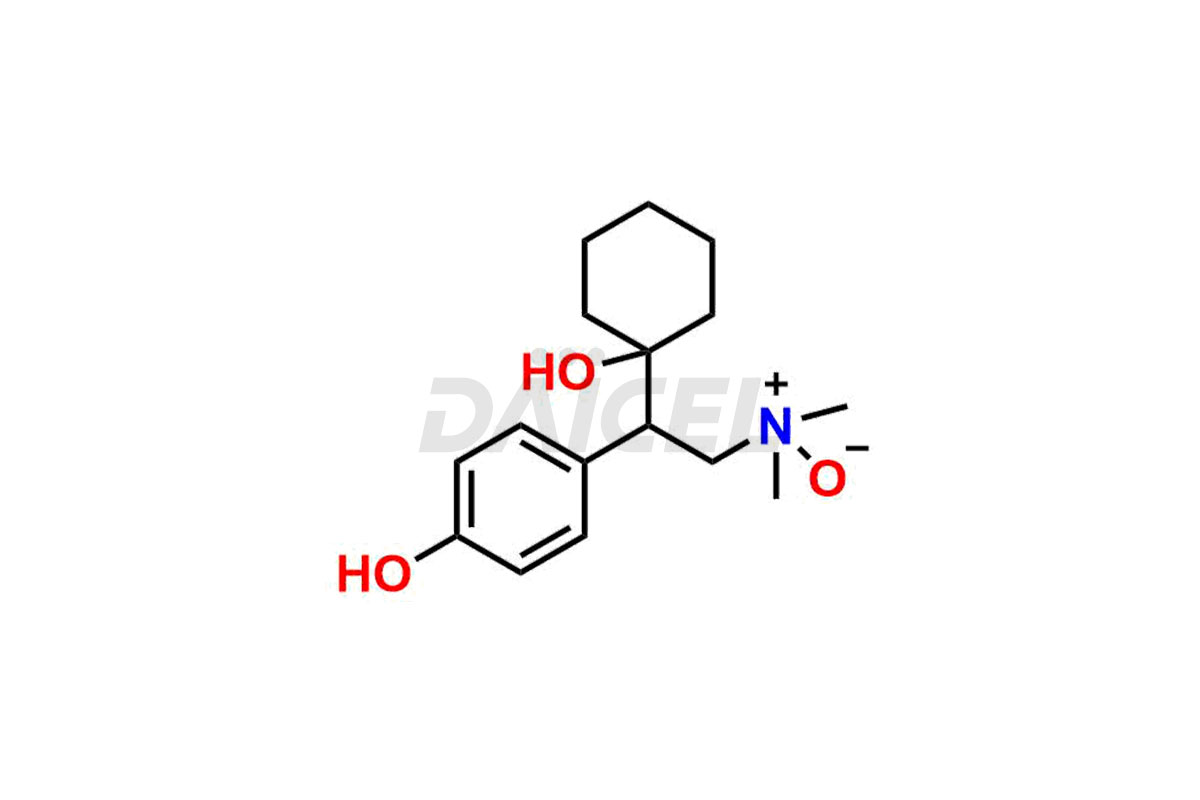
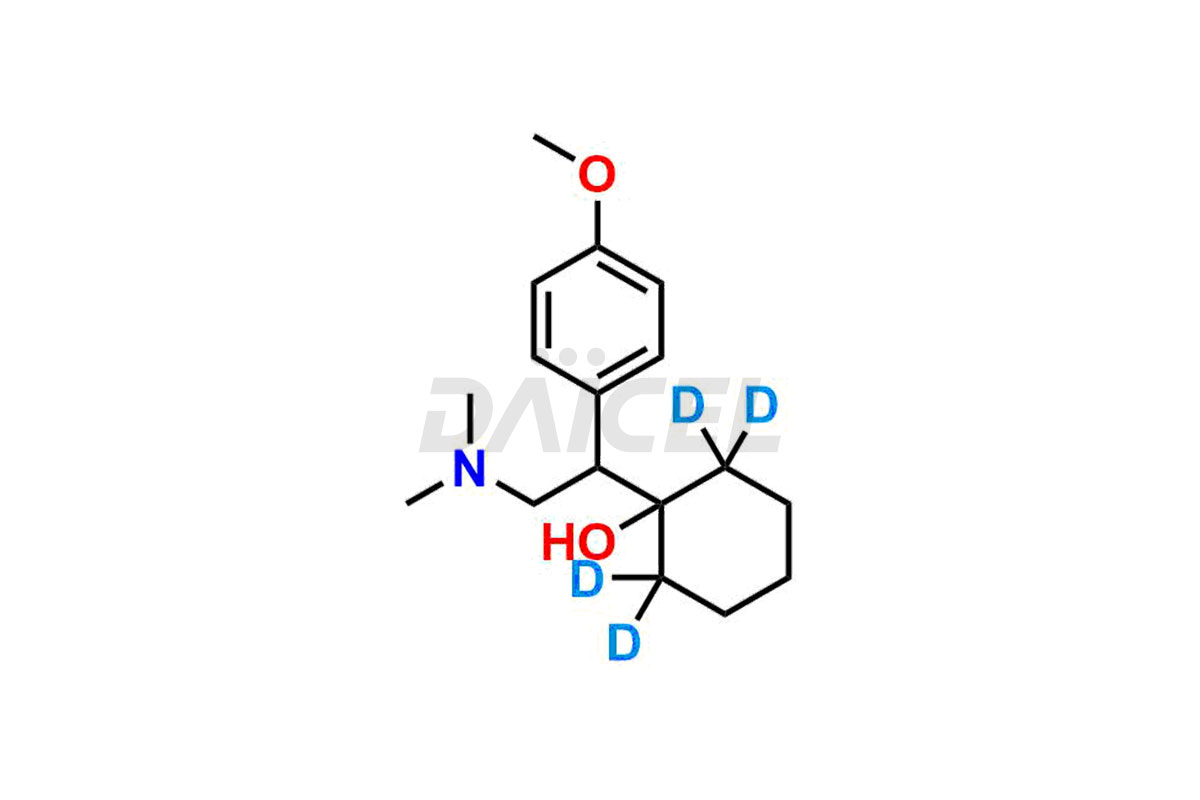
Venlafaxine
General Information
Venlafaxine Impurities and Venlafaxine
Daicel Pharma is a reputable provider of high-quality Venlafaxine impurity standards. The impurities include Des Venlafaxine Impurity-II, Des Venlafaxine-related compound B, O-Desmethyl Venlafaxine, and O-Desmethyl Venlafaxine N-Oxide. These impurities play a crucial role in thoroughly assessing the quality, stability, and safety of Venlafaxine, the active pharmaceutical ingredient. Daicel Pharma also customizes Venlafaxine impurities to meet the specific requirements of clients. Their global shipping capabilities allow customers worldwide to receive these impurities, conveniently ensuring unmatched convenience and reliability.
Venlafaxine [CAS: 93413-69-5] is a serotonin and norepinephrine reuptake inhibitor and an antidepressant. It treats major depressive disorder (MDD), generalized anxiety disorder (GAD), social anxiety disorder (SAD), and panic disorder.
Venlafaxine: Use and Commercial Availability
Venlafaxine treats major depressive disorder, generalized anxiety disorder, social anxiety disorder, and panic disorder. It is an antidepressant medication that helps improve mood and reduce symptoms such as excessive worry, nervousness, and panic attacks. Venlafaxine is commonly prescribed to individuals experiencing these conditions to alleviate their symptoms and enhance their overall well-being.
Venlafaxine is available under the brand names Effexor and Effexor XR.
Venlafaxine Structure and Mechanism of Action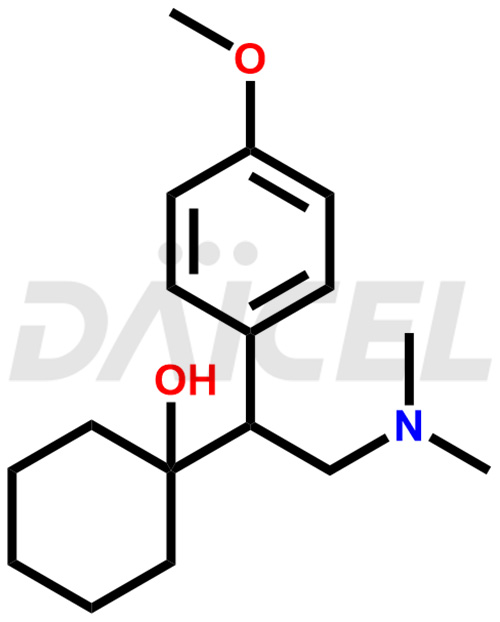
The chemical name of Venlafaxine is 1-[2-(Dimethylamino)-1-(4-methoxyphenyl)ethyl]cyclohexanol. Its chemical formula is C17H27NO2, and its molecular weight is approximately 277.4 g/mol.
Venlafaxine inhibits neuronal serotonin and norepinephrine reuptake and affects neurotransmitter activity in the central nervous system.
Venlafaxine Impurities and Synthesis
Venlafaxine impurities can arise during synthesis1 due to the storage or use of specific raw materials and intermediates in manufacturing. These impurities encompass related compounds, degradation products, and process impurities. Stringent quality control measures and analytical methods are crucial to ensure the purity and safety of Venlafaxine for patient use.
Daicel provides a comprehensive Certificate of Analysis (CoA) for Venlafaxine impurity standards, such as Des Venlafaxine Impurity-II, Des Venlafaxine-related compound B, O-Desmethyl Venlafaxine, and O-Desmethyl Venlafaxine N-Oxide. The CoA includes detailed characterization data such as 1H NMR, 13C NMR, IR, MASS, and HPLC purity2. Additionally, upon delivery, we give 13C-DEPT. Daicel possesses the technology and expertise to synthesize any unknown Venlafaxine impurity or degradation product. We also offer labeled compounds to quantify the efficacy of generic Venlafaxine. For bioanalytical research and BA/BE studies, Daicel supplies O-Desmethyl Venlafaxine-D4 and Venlafaxine-D4, deuterium-labeled standards of Venlafaxine.
References
FAQ's
References
- Husbands, George Edward Morris; Yardley, John Patrick; Muth, Eric Anthony, Phenethylamine Derivatives and Intermediates Therefor, American Home Products Corp., United States, EP112669B1, July 29, 1987
- Vu, Ryan Luan; Helmeste, Daiga; Albers, Lawrence; Reist, Christopher, Rapid determination of venlafaxine and O-desmethylvenlafaxine in human plasma by high-performance liquid chromatography with fluorimetric detection, Journal of Chromatography B: Biomedical Sciences and Applications, Volume: 703, Issue: 1 + 2, Pages: 195-201, 1997
Frequently Asked Questions
What are the challenges in the analysis of venlafaxine impurities?
The challenges faced during Venlafaxine impurity analysis include the sensitivity of analytical methods and the potential variability of impurity levels between batches.
How are Venlafaxine impurities controlled during the manufacturing of the drug?
Impurities in Venlafaxine are controlled during manufacturing with process optimization, careful selection of raw materials, and appropriate analytical testing throughout the production process.
Which solvent helps in analyzing Venlafaxine impurities?
Methanol is a common solvent used to analyze Venlafaxine impurities.
How should Venlafaxine impurities be stored in terms of temperature?
The recommendation is to store Venlafaxine impurities at room temperature, within 2-8 °C.
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.