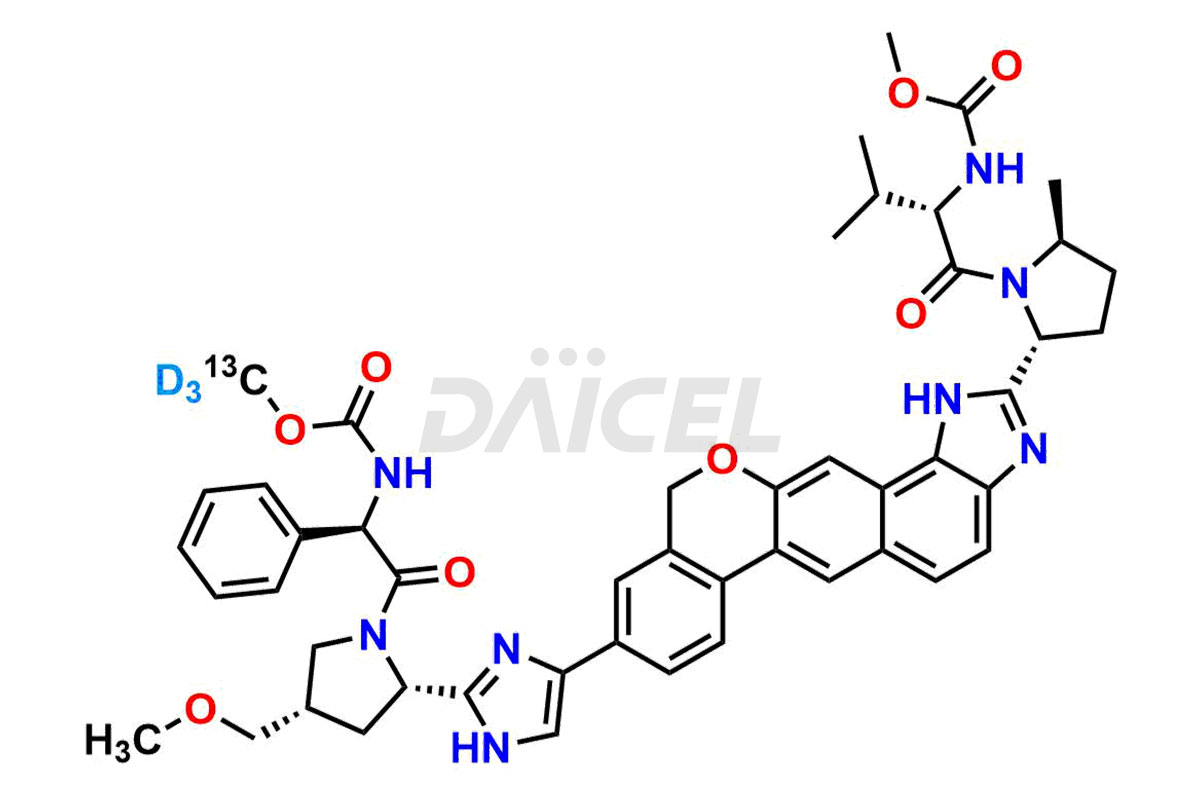
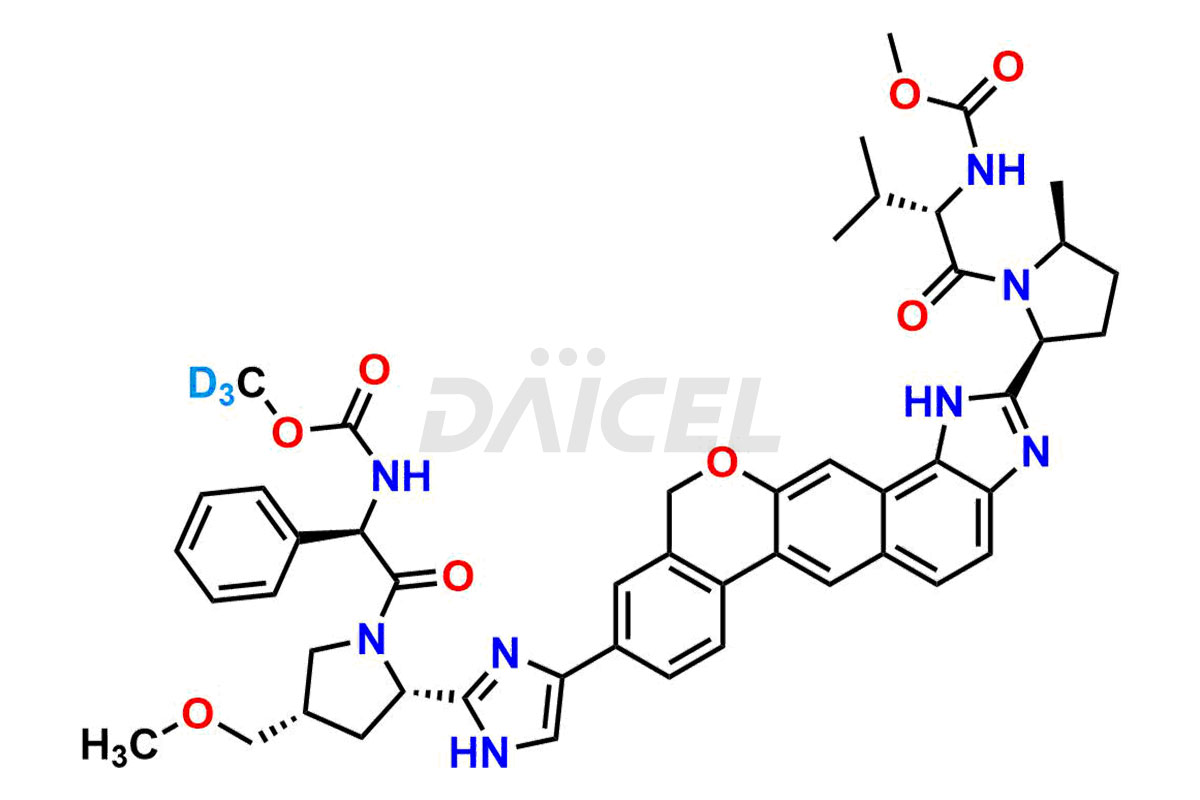
Velpatasvir
General Information
Velpatasvir Impurities and Velpatasvir
Daicel Pharma is a trusted provider of quality Velpatasvir impurity standards. These impurities are crucial in meticulously evaluating the quality, stability, and safety of the active pharmaceutical ingredient, Velpatasvir. Furthermore, Daicel Pharma customizes Velpatasvir impurities, guaranteeing they meet client specifications. With global shipping capabilities, these impurities can be conveniently delivered to customers worldwide, offering unparalleled convenience.
Velpatasvir [CAS: 1377049-84-7] combines with other antiviral medications to treat chronic Hepatitis C Virus (HCV) infection in patients with HCV genotypes 1-6. It also treats HIV-co-infected patients.
Velpatasvir: Use and Commercial Availability
Velpatasvir is a highly effective medication used to treat chronic hepatitis C virus (HCV) infections. It is primarily prescribed with other antiviral drugs, providing comprehensive therapy for HCV genotypes 1-6. Additionally, Velpatasvir is beneficial in managing HCV and HIV co-infection, making it a valuable treatment option for patients with dual infections. Velpatasvir helps improve liver health and overall well-being in individuals with chronic hepatitis C. Velpatasvir alone is currently unavailable under brand names.
Velpatasvir Structure and Mechanism of Action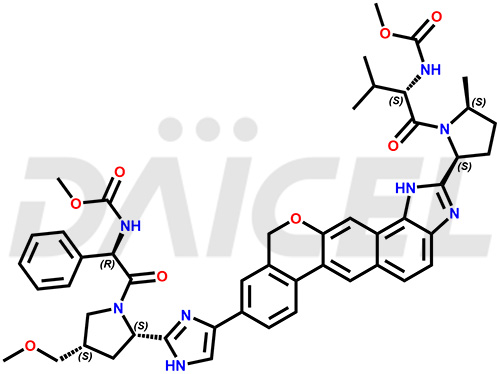
The chemical name of Velpatasvir is Methyl [(2S)-1-[(2S,5S)-2-[9-[2-[(2S,4S)-1-[(2R)-2-[(methoxycarbonyl)amino]-2-phenylacetyl]-4-(methoxymethyl)pyrrolidin-2-yl]-1H-imidazol-5-yl]-1,11-dihydroisochromeno[4′,3′:6,7]naphtho[1,2-d]imidazol-2-yl]-5-methylpyrrolidin-1-yl]-3-methyl-1-oxobutan-2-yl]carbamate. Its chemical formula is C49H54N8O8, and its molecular weight is approximately 883.0 g/mol.
Velpatasvir inhibits the HCV NS5A protein responsible for virus replication.
Velpatasvir Impurities and Synthesis
Velpatasvir impurities can arise during synthesis1 due to the storage or use of specific raw materials and intermediates in manufacturing. These impurities encompass related compounds, degradation products, and process impurities. Stringent quality control measures and analytical methods are crucial to ensure the purity and safety of Velpatasvir for patient use.
Daicel provides a comprehensive Certificate of Analysis (CoA) for Velpatasvir impurity standards. The CoA includes detailed characterization data such as 1H NMR, 13C NMR, IR, MASS, and HPLC purity2. Additionally, upon delivery, we give 13C-DEPT. Daicel possesses the technology and expertise to synthesize any unknown Velpatasvir impurity or degradation product. We also offer labeled compounds to quantify the efficacy of generic Velpatasvir. For bioanalytical research and BA/BE studies, Daicel supplies highly pure Velpatasvir-13CD3 and Velpatasvir-D3, deuterium-labeled compounds of Velpatasvir.
References
FAQ's
References
- Bacon, Elizabeth M.; Cottell, Jeromy J.; Katana, Ashley Anne; Kato, Darryl; Krygowski, Evan S.; Link, John O.; Taylor, James; Tran, Chinh Viet; Trejo Martin, Teresa Alejandra; Yang, Zheng-Yu; et al, Antiviral Compounds, WO2012068234A2, May 24, 2012
- Nalla, Sarath; Seshagiri, Rao J. V. L. N., A stability indicating RP-HPLC method for simultaneous estimation of velpatasvir and sofosbuvir in combined tablet dosage forms, World Journal of Pharmacy and Pharmaceutical Sciences, Volume: 6, Issue: 9, Pages: 1596-1611, 2017
Frequently Asked Questions
Are Velpatasvir impurities tested for potential toxicity?
Yes, impurities in Velpatasvir undergo toxicity testing to assess their potentially harmful effects. The tests help determine their safety profile and establish acceptable limits to ensure patient safety.
How can patients ensure the quality of the Velpatasvir they receive?
Patients can ensure the quality of Velpatasvir by obtaining the medication from reputable sources, such as licensed pharmacies or healthcare providers. Following the prescribed dosage and consulting healthcare professionals during treatment is essential.
Which analytical methods help in analyzing Velpatasvir impurities?
High-performance liquid chromatography (HPLC) is employed to separate and analyze the impurities of Velpatasvir.
How should Velpatasvir impurities be stored in terms of temperature?
The recommendation is to store Velpatasvir impurities at room temperature, within 2-8 °C.
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.