Valproic acid
General Information
Valproic Acid Impurities and Valproic Acid
Daicel Pharma is a trusted provider of quality Valproic Acid impurity standards, including 2,2-Dipropylpentanamide and 2,2-Dipropylpentanenitrile. These impurities are crucial in meticulously evaluating the quality, stability, and safety of the active pharmaceutical ingredient, Valproic Acid. Furthermore, Daicel Pharma specializes in customizing Valproic Acid impurities, guaranteeing that it meets client specifications. With global shipping capabilities, these impurities can be conveniently delivered to customers worldwide, offering unparalleled convenience.
Valproic Acid [CAS: 99-66-1] is an anticonvulsant and a mood stabilizer. It treats various conditions like epilepsy, bipolar disorders, and migraines.
Valproic Acid: Use and Commercial Availability
Valproic Acid is an anticonvulsant that effectively treats seizures associated with epilepsy and other convulsions. Additionally, Valproic acid has proven efficacy in managing migraines, helping to reduce their frequency and intensity. It treats manic episodes associated with bipolar disorders.
Valproic Acid is available under the brand names Depakene and Stavzor.
Valproic Acid Structure and Mechanism of Action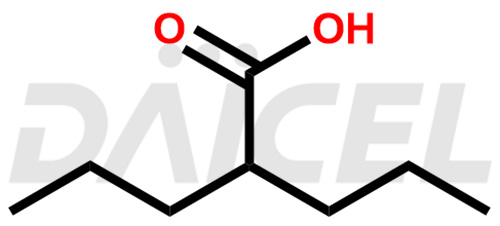
The chemical name of Valproic Acid is 2-Propylpentanoic acid. Its chemical formula is C8H16O2, and its molecular weight is approximately 144.21 g/mol.
The mechanism of action of Valproic Acid is not known.
Valproic Acid Impurities and Synthesis
Valproic Acid impurities can arise during synthesis1 due to the storage or use of raw materials and intermediates in manufacturing. These impurities encompass related compounds, degradation products, and process impurities. Stringent quality control measures and analytical methods are crucial to ensure the purity and safety of Valproic Acid for patient use.
Daicel Pharma provides a comprehensive Certificate of Analysis (CoA) for Valproic Acid impurity standards, such as 2,2-Dipropylpentanamide and 2,2-Dipropylpentanenitrile. The CoA includes detailed characterization data such as 1H NMR, 13C NMR, IR, MASS, and HPLC purity2. Additionally, upon delivery, we give 13C-DEPT. Daicel possesses the technology and expertise to synthesize any unknown Valproic Acid impurity or degradation product.
References
FAQ's
References
- Chignac, Michel; Grain, Claude; Pigerol, Charles, Process for the preparation of acetic acid derivatives, Labaz S. A., France, US4127604A, November 28, 1978
- Jensen, Chris J.; Gugler, Roland, Sensitive gas-liquid chromatographic method for determination of valproic acid in biological fluids, Journal of Chromatography Volume: 137, Issue: 1, Pages: 188-93, 1977
Frequently Asked Questions
How can the presence of impurities in Valproic Acid be minimized?
By adhering to strict quality control measures, impurities in Valproic Acid are minimized. These measures include following good manufacturing practices (GMP), proper storage conditions, and using reliable suppliers for raw materials.
Which solvent helps in analyzing Valproic Acid impurities?
Acetonitrile is the common solvent used for analyzing many Valproic Acid impurities.
Which analytical methods help analyze Valproic Acid impurities?
The UPLC method is employed to separate and analyze the impurities of Valproic Acid.
How should Valproic Acid impurities be stored in terms of temperature?
The recommendation is to store Valproic Acid impurities at room temperature, within 2-8 °C.
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.



