LOAD MORE
You're viewed 9 of 17 products
Daicel Pharma is a reliable supplier of high-quality Ulipristal impurity standards, encompassing Diketal intermediate-1, Diketal intermediate-2, Diketal intermediate-3, Ulipristal Acetate Metabolite (UPA+2H), Ulipristal acetate N-Oxide, Ulipristal Impurity-3, and more. The presence of these impurities is crucial in conducting a comprehensive assessment of the quality, stability, and safety of active pharmaceutical ingredients. Daicel Pharma synthesizes Ulipristal impurities to meet clients’ needs. With the ability to ship globally, customers worldwide can conveniently receive these impurities.
Ulipristal [CAS: 159811-51-5] is a derivative of 19-norprogestin that functions as a selective modulator of progesterone receptors, primarily exhibiting antiprogestin properties.
Ulipristal is an emergency contraceptive or the “morning-after pill.” It effectively prevents pregnancy when taken within a specific timeframe after unprotected sexual intercourse or contraceptive failure. Additionally, it is for treating uterine fibroids and noncancerous growths in the uterus. Ulipristal offers a reliable option for emergency contraception and managing uterine fibroids. Ulipristal is available under the brand name Ella. It contains the active ingredient, Ulipristal.
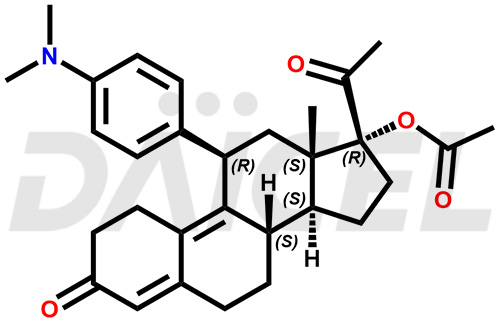
The chemical name of Ulipristal is (11β)-11-[4-(Dimethylamino)phenyl]-17-hydroxy-19-norpregna-4,9-diene-3,20-dione. Its chemical formula is C28H35NO3, and its molecular weight is approximately 433.6 g/mol.
Ulipristal inhibits or delays ovulation and postpones follicular rupture.
Ulipristal impurities can arise during synthesis1 due to storage or using specific raw materials and intermediates in preparation. These impurities encompass related compounds, degradation products, and process impurities. Stringent quality control measures and analytical methods are crucial to ensure the purity and safety of Ulipristal for patient use.
Daicel provides a comprehensive Certificate of Analysis (CoA) for Ulipristal impurity standards such as Diketal intermediate-1, Diketal intermediate-2, Diketal intermediate-3, Ulipristal Acetate Metabolite (UPA+2H), Ulipristal acetate N-Oxide, Ulipristal Impurity-3, and more. The CoA includes detailed characterization data such as 1H NMR, 13C NMR, IR, MASS, and HPLC purity. Additionally, upon delivery, we give a 13C-DEPT. Daicel possesses the technology and expertise to synthesize any unknown Ulipristal impurity or degradation product. We also offer labeled compounds to quantify the efficacy of generic Ulipristal. For bioanalytical research and BA/BE studies, Daicel supplies highly pure N-Desmethyl Ulipristal Acetate-D3, Ulipristal acetate -D3(acyl-D3), Ulipristal acetate-D3, Ulipristal-D3, deuterium-labeled Ulipristal impurity standards.
Yes, purification methods such as chromatography, filtration, and crystallization can eliminate impurities from Ulipristal. These techniques aid in separating and isolating impurities, resulting in a purified form of Ulipristal.
Impurities in Ulipristal are identified through analytical techniques, such as HPLC.
Acetonitrile is the solvent used to analyze impurities in Ulipristal.
Ulipristal Impurities should be stored at a controlled room temperature between 2-8°C or as indicated on the Certificate of Analysis (CoA).
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.