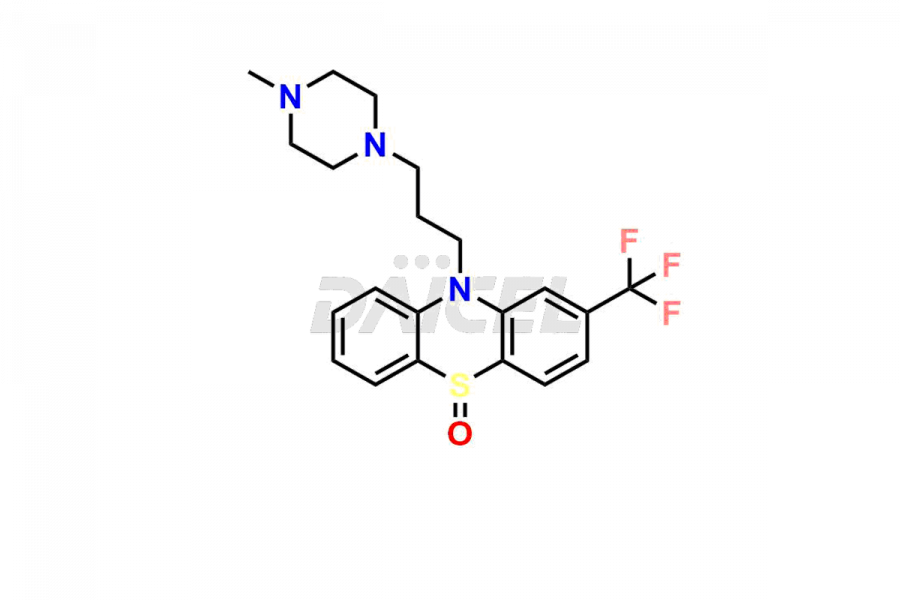
Trifluoperazine
General Information
Trifluoperazine Impurities and Trifluoperazine
Daicel Pharma is a trusted provider known for synthesizing premium-grade Trifluoperazine impurity standards like 10-(3-(4-methylpiperazin-1-yl)propyl)-2-(trifluoromethyl)-10H-phenothiazine 5,5-dioxide and Trifluoperazine Sulfoxide. These impurities are crucial in assessing the Trifluoperazine quality, stability, and safety. Moreover, Daicel Pharma offers a tailored synthesis of Trifluoperazine impurities, catering to clients’ specific requirements worldwide and ensuring prompt delivery and customer satisfaction.
Trifluoperazine [CAS: 117-89-5] is a phenothiazine and antipsychotic agent used to treat anxiety disorders and depressive symptoms secondary to anxiety and agitation. It also treats mental illnesses, including schizophrenia and psychotic disorders. It prevents hallucinations and delusions.
Trifluoperazine: Use and Commercial Availability
Trifluoperazine is a potent antipsychotic medication known for its effectiveness. It possesses robust anticonvulsant properties and is extensively employed in psychiatry to treat conditions such as schizophrenia and other mental disorders.
Trifluoperazine is available under brand names such as Stelazine, which contains the active ingredient, Trifluoperazine.
Trifluoperazine Structure and Mechanism of Action 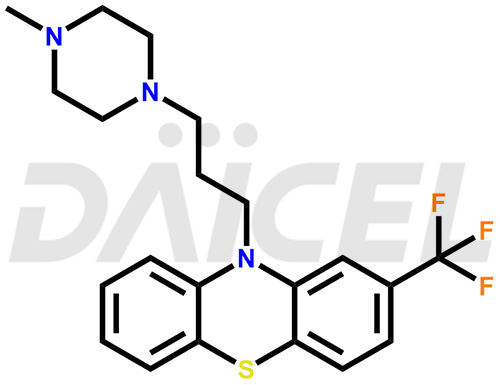
The chemical name of Trifluoperazine is 10-[3-(4-Methyl-1-piperazinyl)propyl]-2-(trifluoromethyl)-10H-phenothiazine. Its chemical formula is C21H24F3N3S, and its molecular weight is approximately 407.5 g/mol
Trifluoperazine inhibits postsynaptic mesolimbic dopaminergic D1 and D2 receptors in the brain.
Trifluoperazine Impurities and Synthesis
Trifluoperazine preparation1 process can develop impurities, which could diminish its effectiveness. These impurities may come from various raw materials, intermediates, and chemicals used in synthesizing Trifluoperazine, among other sources. It is necessary to manage and monitor these impurities closely to ensure the drug’s efficacy and safety.
Daicel offers a Certificate of Analysis (CoA) from a cGMP-compliant analytical facility for Trifluoperazine impurity standards such as 10-(3-(4-methylpiperazin-1-yl)propyl)-2-(trifluoromethyl)-10H-phenothiazine 5,5-dioxide and Trifluoperazine Sulfoxide. The certificate of analysis (CoA) includes comprehensive characterization data, such as 1H NMR, 13C NMR, IR, MASS, and HPLC purity2. We give 13C-DEPT upon request. A complete characterization report is provided upon delivery. Daicel possesses the technology and expertise to synthesize any unknown Trifluoperazine impurity or degradation product.
References
FAQ's
References
- Craig, P. N.; Nodiff, E. A.; Lafferty, J. J.; Ullyot, G. E., New (trifluoromethyl)phenothiazine derivatives, Journal of Organic Chemistry, Volume: 22, Pages: 709-11, 1957
- Watson, James Robert; Matsui, Fumi; French, Warren N., Trifluoperazine tablets: alternative methods of analysis, Journal of Pharmaceutical Sciences, Volume: 59, Issue: 3, Pages: 391-4 1970
Frequently Asked Questions
How can impurity profiling contribute to Trifluoperazine preparation processes?
Impurity profiling significantly improves Trifluoperazine preparation processes by identifying the underlying factors responsible for impurity formation. It enhances the overall quality of Trifluoperazine products.
What are the potential consequences of exceeding impurity limits in Trifluoperazine products?
Exceeding impurity limits in Trifluoperazine products can have serious consequences, including compromised product quality, reduced therapeutic efficacy, increased risk of adverse effects, and regulatory non-compliance.
How are Trifluoperazine impurities identified and characterized?
Trifluoperazine impurities are identified and characterized using analytical techniques such as High-performance Liquid chromatography (HPLC). These techniques help determine impurities' chemical structure, concentration, and potential toxicity.
What are the temperature conditions required to store Trifluoperazine impurities?
Trifluoperazine impurities should be stored at a controlled room temperature between 2-8°C or as indicated on the Certificate of Analysis (CoA).
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.