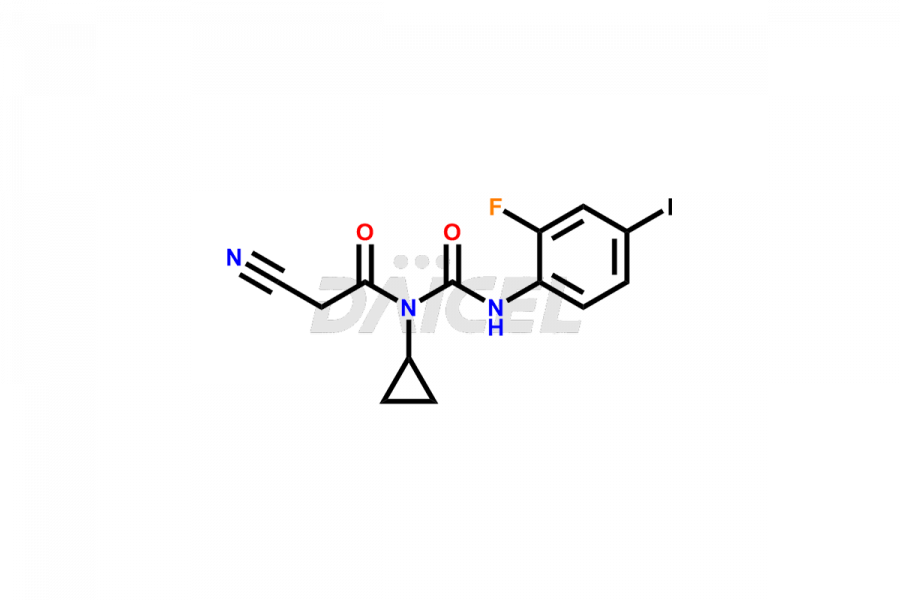
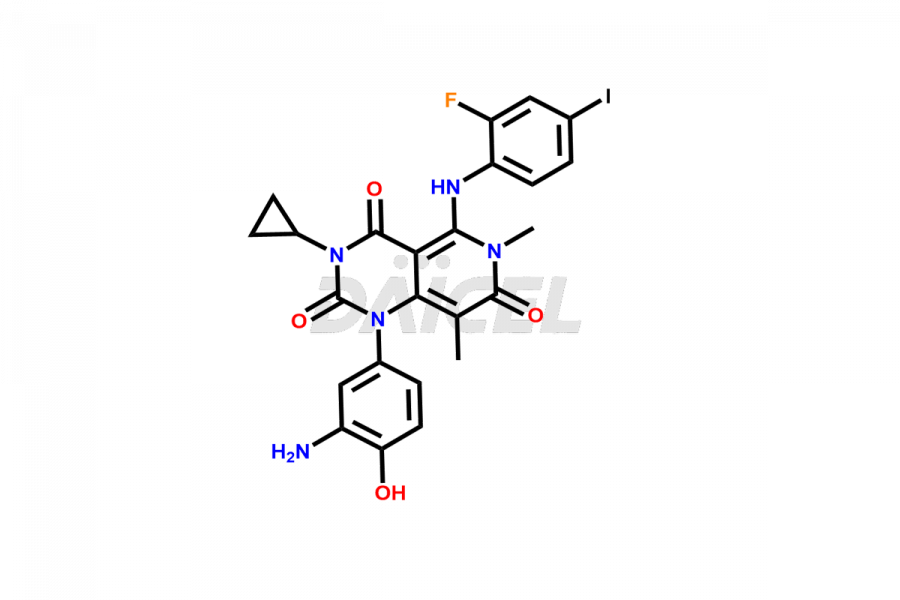
Trametinib
General Information
Trametinib Impurities and Trametinib
Daicel Pharma offers a selective range of exclusive M7 Metabolite of Trametinib and M5 Metabolite of Trametinib. Impurities are of utmost importance when assessing the quality, stability, and biological safety of the active pharmaceutical ingredient, Trametinib. Daicel Pharma specializes in synthesizing custom Trametinib impurities to meet precise customer specifications. With a commitment to worldwide delivery, Daicel Pharma ensures that customers receive the synthesized Trametinib impurities promptly and reliably.
Trametinib [CAS: 871700-17-3] is an inhibitor of mitogen-activated extracellular signal-regulated kinase 1 (MEK1) and MEK2, taken orally. It helps to treat several cancers with BRAF mutations.
Trametinib: Use and Commercial Availability
Trametinib targets and inhibits the activity of MEK1 and MEK2 enzymes. It can be used as a monotherapy or in combination with dabrafenib, a BRAF inhibitor, to enhance the effectiveness of the treatment. Cancers are treated with this medication, including non-small cell lung and thyroid cancer. Trametinib is available under the brand name Mekinist, which contains the active ingredient, Trametinib.
Trametinib Structure and Mechanism of Action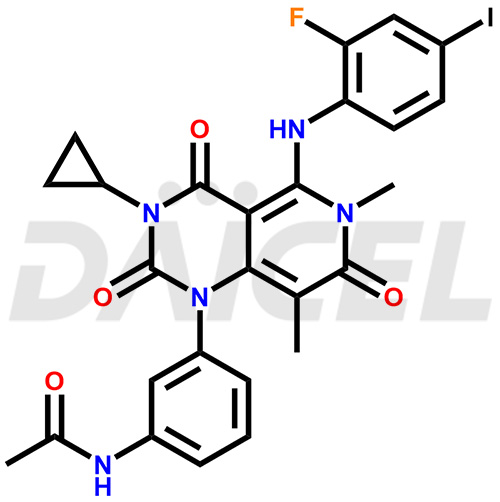
The chemical name of Trametinib is N-[3-[3-Cyclopropyl-5-[(2-fluoro-4-iodophenyl)amino]-3,4,6,7-tetrahydro-6,8-dimethyl-2,4,7-trioxopyrido[4,3-d]pyrimidin-1(2H)-yl]phenyl]acetamide. Its chemical formula is C26H23FIN5O4, and its molecular weight is approximately 615.4 g/mol.
Trametinib is a highly selective, reversible inhibitor of MEK1 (mitogen-activated extracellular signal-regulated kinase 1) and MEK2 responsible for BRAF V600E mutations.
Trametinib Impurities and Synthesis
During the synthesis1 and storage of Trametinib, impurities can arise, including related substances, degradation products, and residual solvents. It is essential to carefully monitor and control these impurities to ensure the medication’s safety, efficacy, and overall quality.
Daicel Pharma offers a comprehensive Certificate of Analysis (CoA) for impurity standards such as – M7 Metabolite of Trametinib and M5 Metabolite of Trametinib. They are synthesized in compliance with current Good Manufacturing Practices (cGMP). The CoA includes detailed characterization data, such as 1H NMR, 13C NMR, IR, MASS, and HPLC purity, providing a thorough understanding of the impurity profile2, 3. Upon request, Daicel can also provide 13C-DEPT data for further characterization. Furthermore, Daicel Pharma possesses the technical expertise to synthesize any unknown impurities or degradation products of Trametinib. By leveraging this capability, customers gain convenient access to elemental impurities required for their research and development endeavors.
References
FAQ's
References
- Sakai, Toshiyuki; Kawasaki, Hisashi; Abe, Hiroyuki; Hayakawa, Kazuhide; Iida, Tetsuya; Kikuchi, Shinichi; Yamaguchi, Takayuki; Nanayama, Toyomichi; Kurachi, Hironori; Tamaru, Masahiro; et al, 5-Amino-2,4,7-Trioxo-3,4,7,8-Tetrahydro-2h-Pyrido[2,3-D]Pyrimidine Derivatives And Related Compounds For The Treatment Of Cancer, Japan Tobacco Inc., Japan, EP1761528B1, January 9, 2008
- Madhuresh K. Sethi*, Sanjay Mahajan, Bhairaiah Mara, Upendranath Veera, Anitha Nimmagadda and Purbita Chakraborty, Identification and synthesis of impurities formed during Trametinib Dimethyl sulfoxide preparation, Der Pharmacia Lettre, 2016, 8 (6):321-327
- Nijenhuis, C. M.; Haverkate, H.; Rosing, H.; Schellens, J. H. M.; Beijnen, J. H., Simultaneous quantification of dabrafenib and trametinib in human plasma using high-performance liquid chromatography-tandem mass spectrometry, Journal of Pharmaceutical and Biomedical Analysis Volume: 125, Pages: 270-279, 2016
Frequently Asked Questions
How are Trametinib impurities detected and quantified?
Analytical Methods such as High-Performance Liquid Chromatography (HPLC) can detect impurities in Trametinib.
Can Trametinib impurities affect patient safety?
Yes, impurities in Trametinib can impact patient safety. Depending on their nature and concentration, contaminants can cause adverse effects or reduce the efficacy of the medication.
Which solvents help in the analysis of Trametinib impurities?
Methanol achieves optimal solubility and separation of Trametinib impurities.
What are the temperature conditions required to store Trametinib impurities?
Trametinib impurities should generally be stored at a controlled room temperature between 2-8 ⁰C or as indicated on the Certificate of Analysis (CoA).
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.