Timolol
General Information
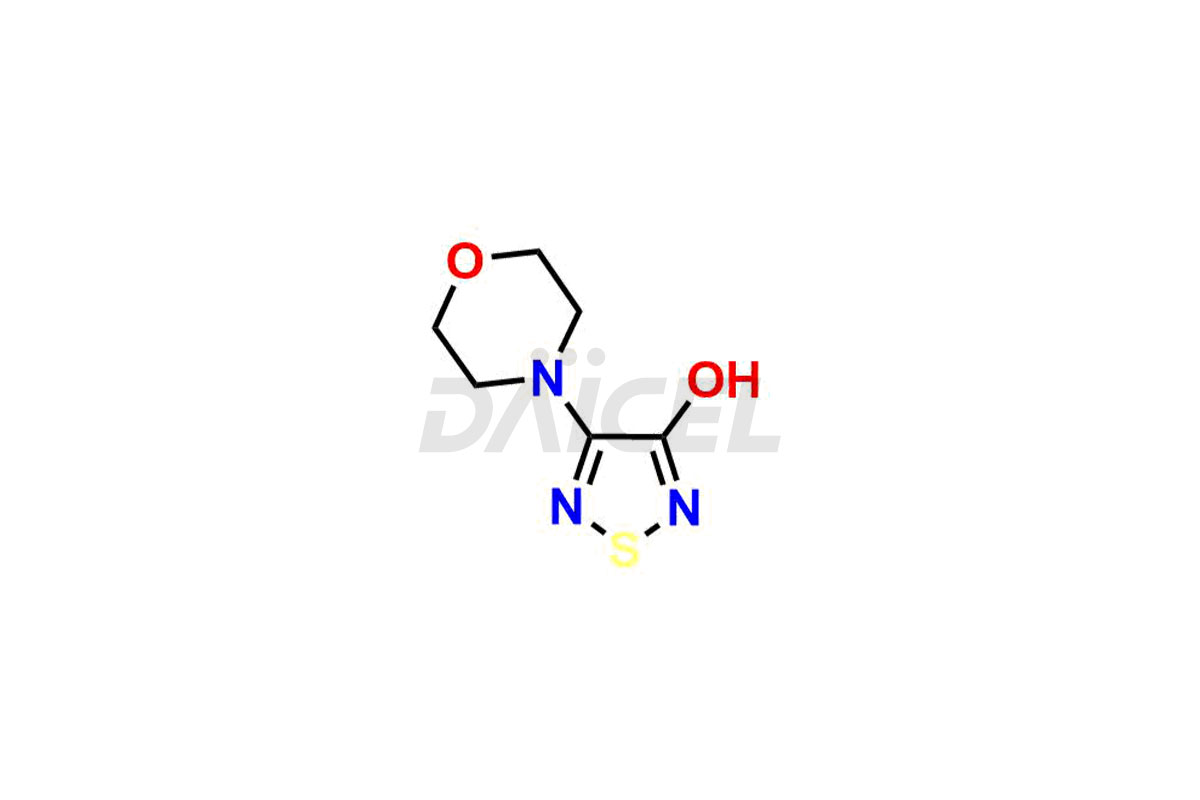
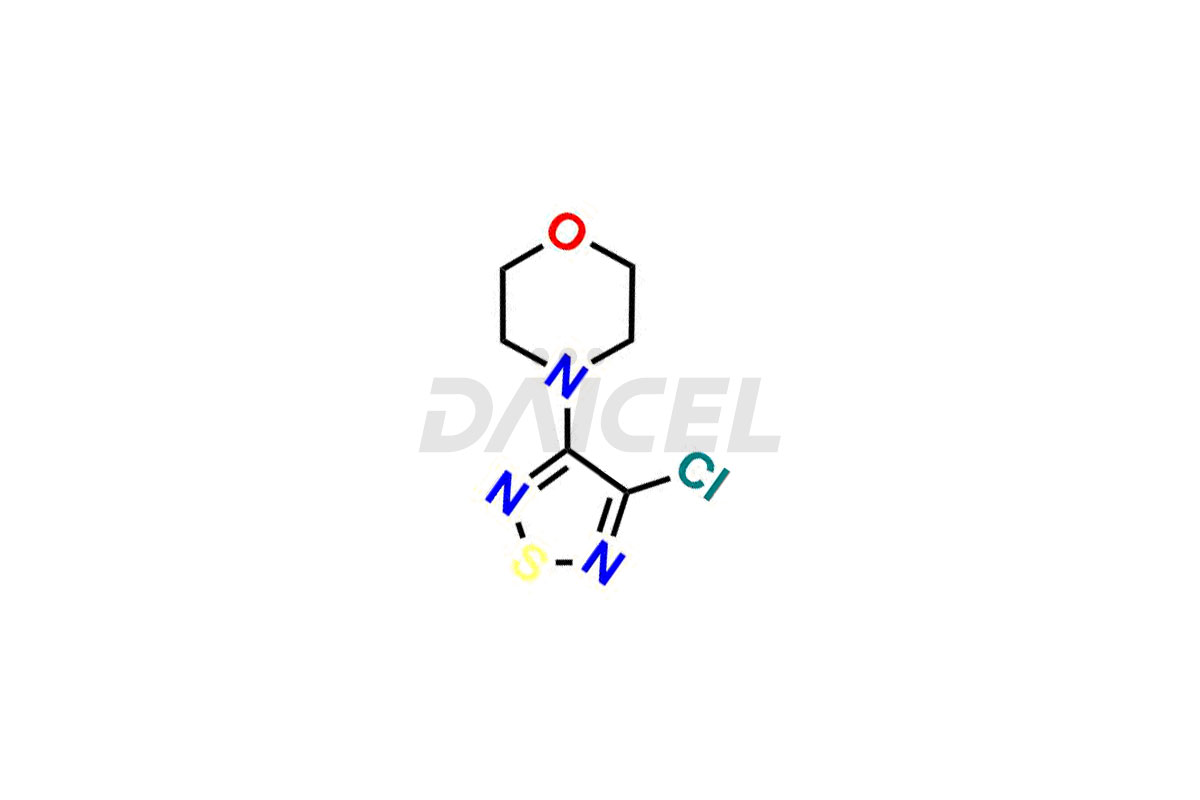
Timolol Impurities and Timolol
Daicel Pharma is a trusted provider of vital Timolol impurities, including R-Timolol, Timolol Impurity-D, and Timolol Impurity-F. These impurities are crucial in assessing the quality, stability, and biological safety of the active pharmaceutical ingredient, Timolol. Moreover, Daicel Pharma excels in tailoring the synthesis of Timolol impurities to meet individual client specifications. With worldwide shipping facilities, customers can enjoy the convenience and flexibility of accessing these high-quality impurities.
Timolol [CAS: 26839-75-8] is a nonselective beta-adrenergic receptor blocker that treats and manages open-angle glaucoma and ocular hypertension.
Timolol: Use and Commercial Availability
Timolol treats various conditions such as ocular hypertension, angina pectoris, and the prevention of vascular headaches. It manages elevated intraocular pressure in patients with ocular hypertension or open-angle glaucoma. It also prevents migraine headaches.
Timolol is available under Betimol, Blocadren, Istalol, etc., which contain the active ingredient, Timolol.
Timolol Structure and Mechanism of Action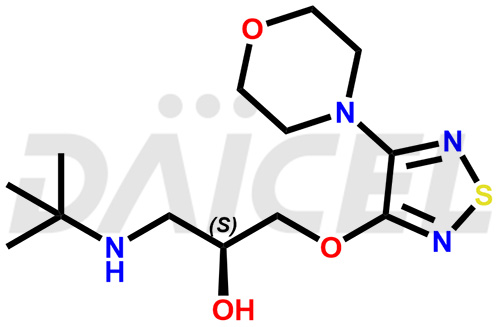
The chemical name of Timolol is (2S)-1-[(1,1-Dimethylethyl)amino]-3-[[4-(4-morpholinyl)-1,2,5-thiadiazol-3-yl]oxy]-2-propanol. Its chemical formula is C13H24N4O3S, and its molecular weight is approximately 316.42 g/mol.
The exact mechanism of action of Timolol is unknown.
Timolol Impurities and Synthesis
In manufacturing1 Timolol, impurity formation is possible, which impacts its effectiveness. They may originate from different sources, such as raw materials, intermediates, and chemicals for synthesizing Timolol. It is crucial to closely manage and monitor these impurities to safeguard the optimal efficacy and safety of the medication.
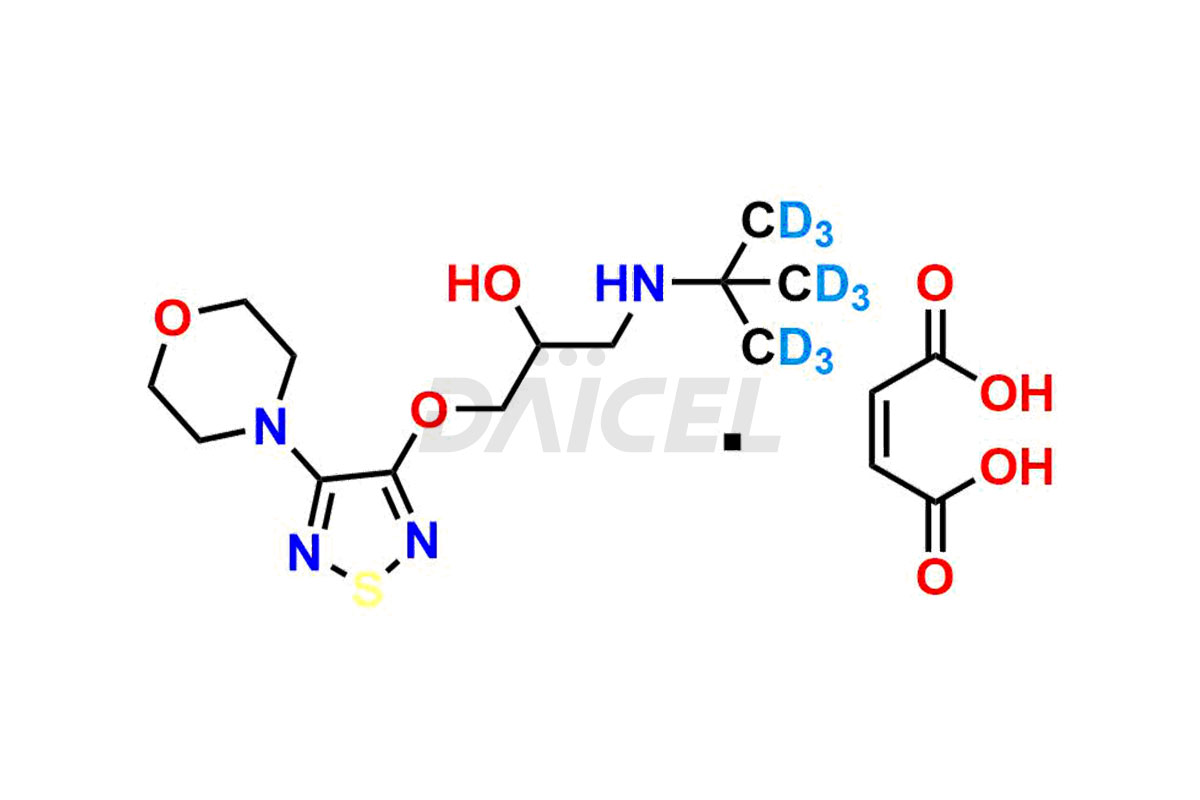
Daicel offers a wholesome and integrated Certificate of Analysis (CoA) for Timolol impurity standards, encompassing R-Timolol, Timolol Impurity-D, and Timolol Impurity-F. The CoA provides detailed characterization data, including 1H NMR, 13C NMR, IR, MASS, and HPLC purity2. Additionally, we give a detailed 13C-DEPT upon delivery. With advanced technology and expertise, Daicel can synthesize any unknown Timolol impurity or degradation product. We also supply labeled compounds, facilitating the quantification of generic Timolol’s efficacy. For bioanalytical research and BA/BE studies, Daicel offers Timolol-D9 Maleate, deuterium-labeled Timolol impurity standards.
References
FAQ's
References
- Wasson, Burton K., 4-(3-Secondary Amino-2-Hydroxy-Proxy) 1 2 5-Thiadiazoles, Frosst, Charles E., and Co., US3655663A, April 11, 1972
- Mohamed, M. E.; Tawakkol, M. S.; Aboul-Enein, H. Y., Spectrophotometric determination of timolol and other β-adrenergic blocking drugs and in pharmaceutical preparations, Spectroscopy Letters, Volume: 15, Issue: 8, Pages: 609-21, 1982
Frequently Asked Questions
What are the common impurities that can occur during the manufacturing of Timolol?
Common impurities that may occur during the manufacturing of Timolol include truncated or modified peptide chains, residual solvents, heavy metals, and related substances.
How are Timolol impurities identified and quantified during the manufacturing process?
Timolol impurities are identified and quantified during manufacturing using techniques like UPLC. These techniques help determine the type and quantity of impurities in the sample.
Can impurities in Timolol impact its bioavailability?
Timolol impurities can affect its bioavailability and potentially lead to reduced absorption or altered pharmacokinetics.
What measures prevent the formation of Timolol impurities during drug storage?
Timolol is stored under carefully controlled conditions, including temperature and humidity control, to prevent the formation of impurities.
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.