Tigecycline
General Information
Tigecycline Impurities and Tigecycline
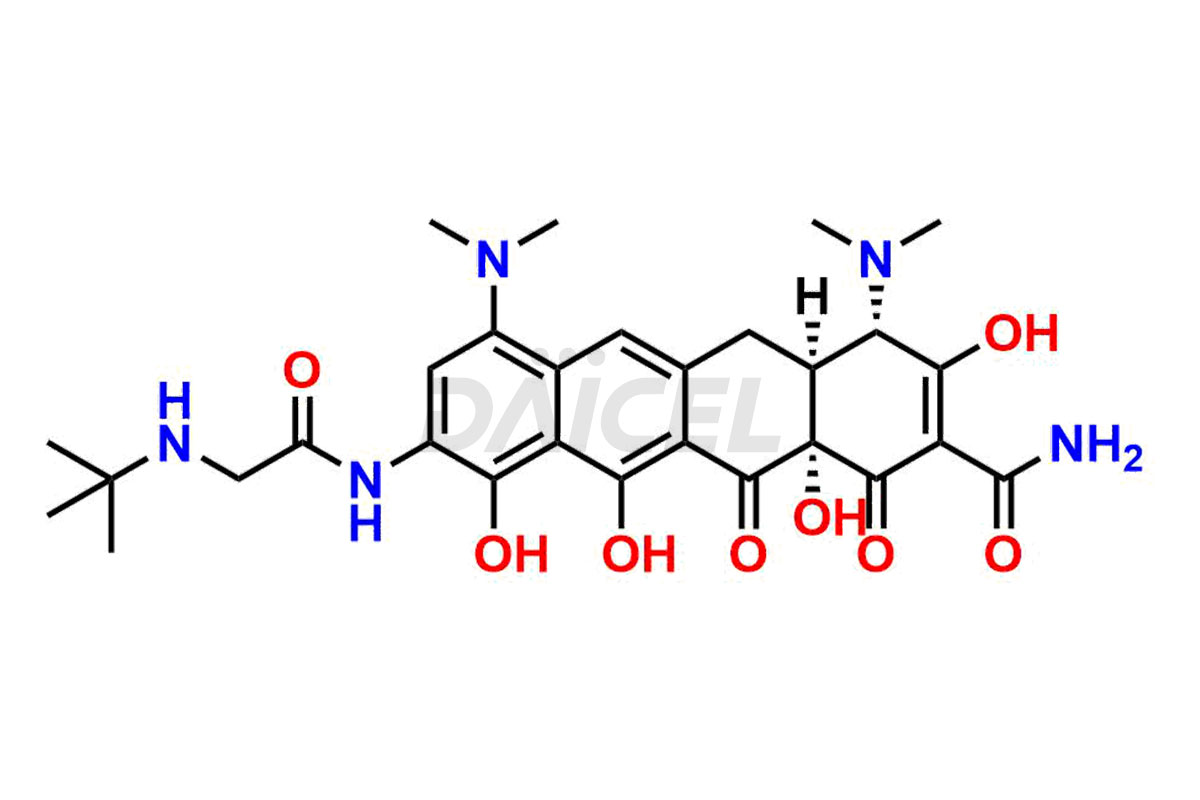
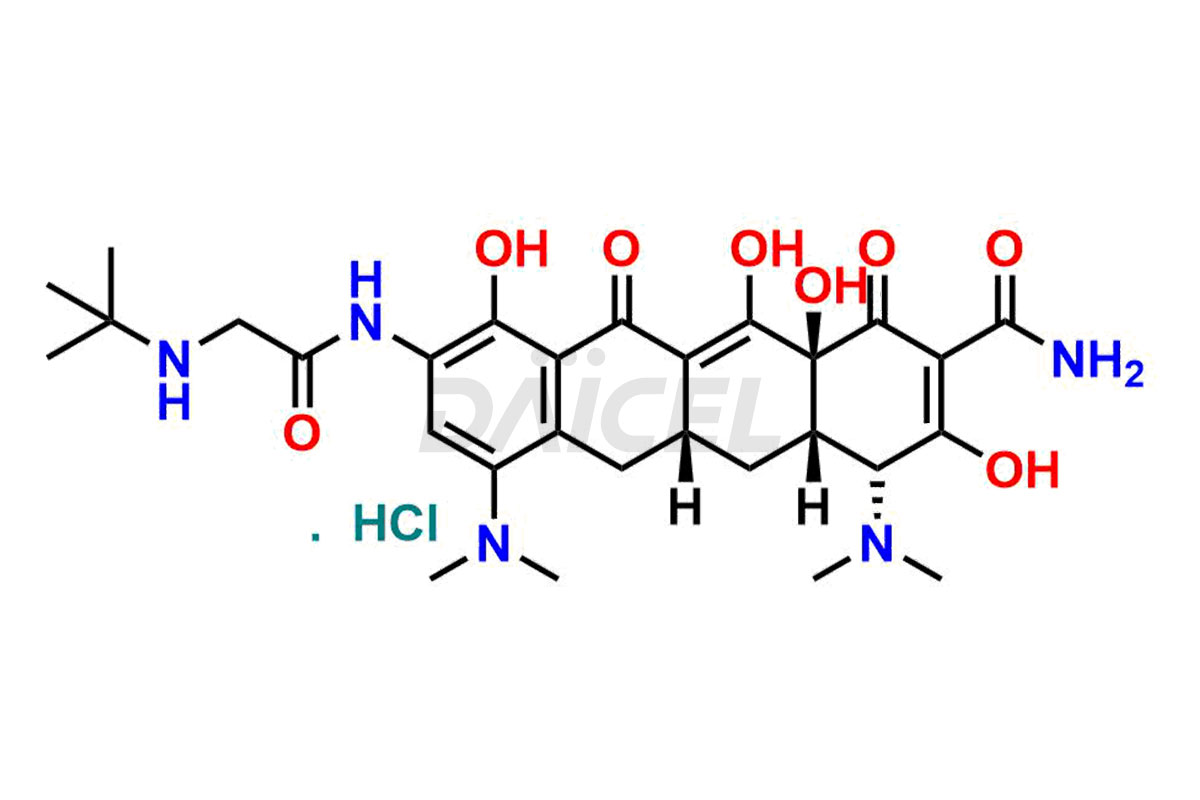
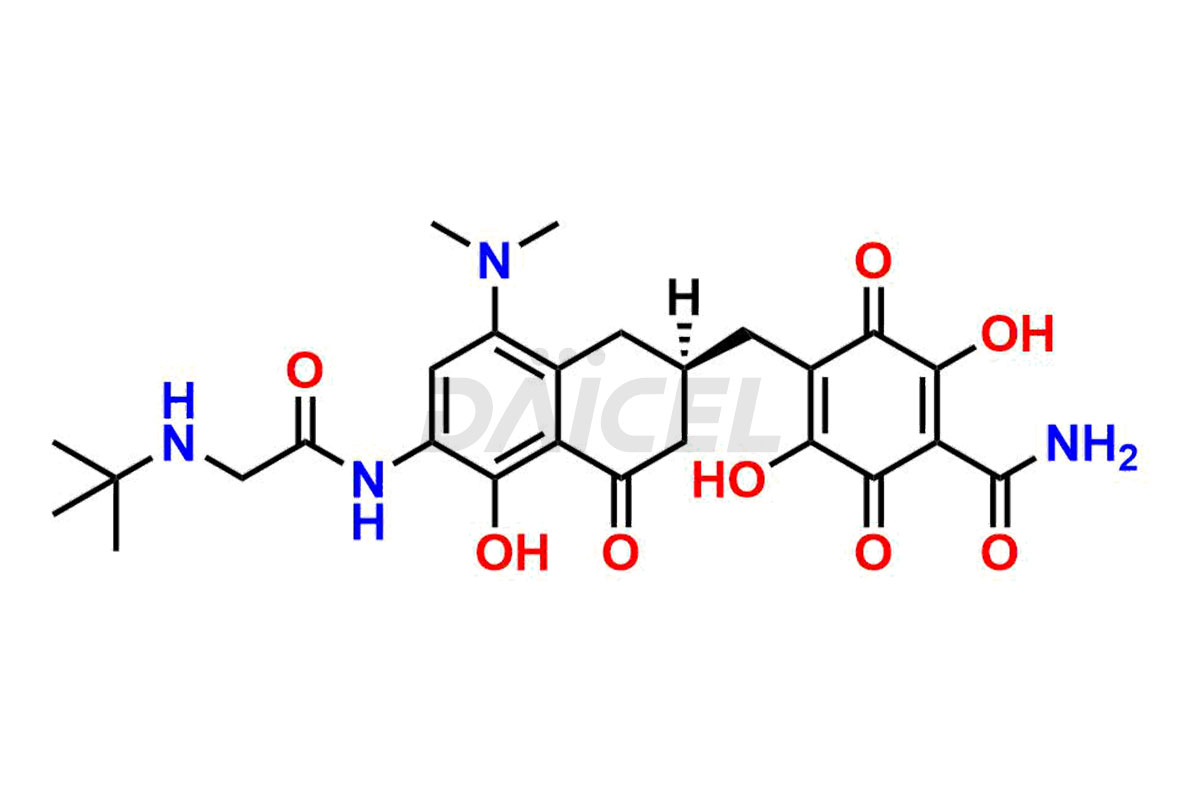
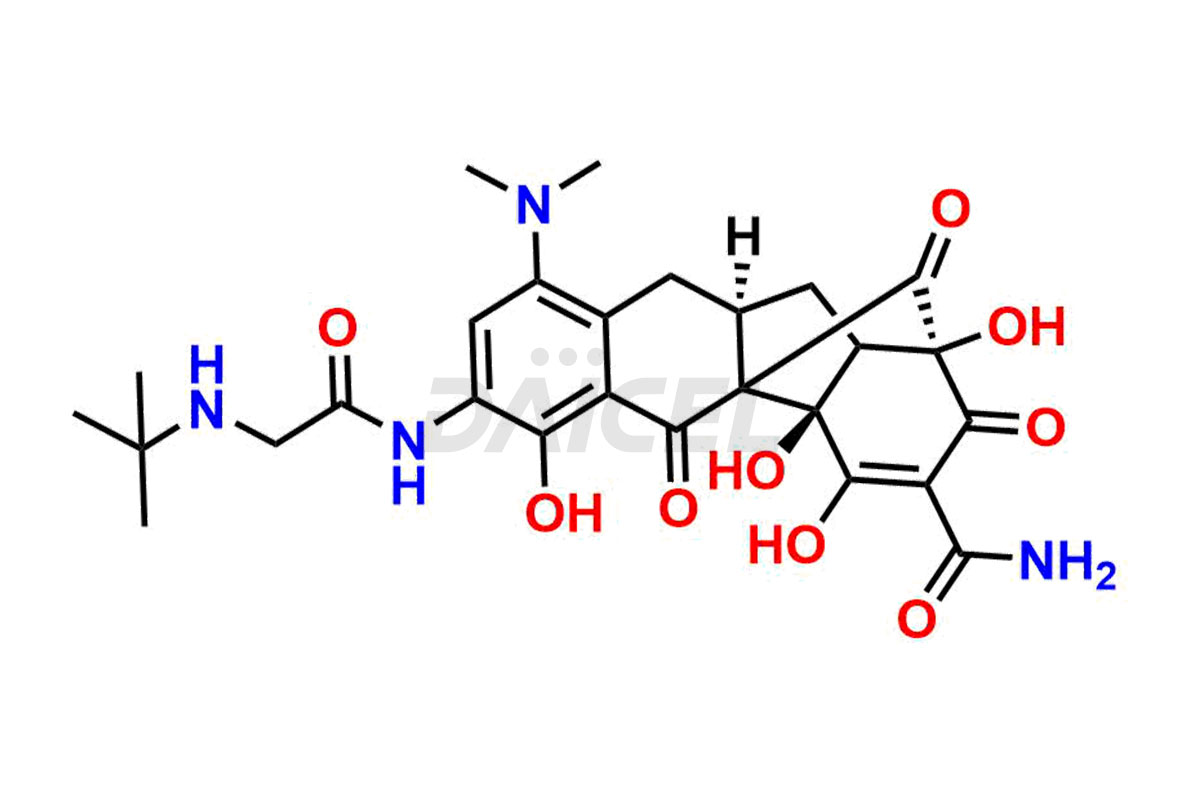
Daicel Pharma is a reliable source for synthesizing high-quality Tigecycline impurities, specifically 12-oxo-11 hydroxy Tigecycline, 4-Epimer of Tigecycline Hydrochloride, Tigecycline quinone Analog, and Tigecycline Tricyclic Analog. These impurities are essential for analyzing the quality, stability, and biological safety of the active pharmaceutical ingredient, Tigecycline. Additionally, Daicel Pharma specializes in the custom synthesis of Tigecycline impurities, catering to specific client requirements. These high-quality impurities can be shipped globally, offering convenience and flexibility to customers worldwide.
Tigecycline [CAS: 220620-09-7] is an intravenous glycylcycline antibiotic that treats Complicated skin and soft tissue infections (cSSTI).
Tigecycline: Use and Commercial Availability
Tigecycline is the first drug in the glycylcycline class of antibiotics. It helps treat infections caused by susceptible organisms in two conditions: complicated skin and skin structure infections (cSSSI) and complicated intraabdominal infections.
Tigecycline is available under the brand Tygacil which contains the active ingredient, Tigecycline.
Tigecycline Structure and Mechanism of Action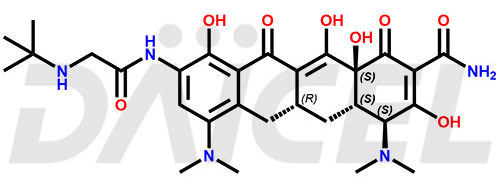
The chemical name of Tigecycline is (4S,4aS,5aR,12aS)-4,7-Bis(dimethylamino)-9-[[2-[(1,1-dimethylethyl)amino]acetyl]amino]-1,4,4a,5,5a,6,11,12a-octahydro-3,10,12,12a-tetrahydroxy-1,11-dioxo-2-naphthacenecarboxamide. Its chemical formula is C29H39N5O8, and its molecular weight is approximately 585.6 g/mol.
Tigecycline prevents protein replication in bacteria binding to its ribosomes. It blocks the incorporation of amino acid residues into elongating peptide chains.
Tigecycline Impurities and Synthesis
During Tigecycline’s manufacturing1, impurity formation is possible and can compromise its effectiveness. They can arise from various sources, including the raw materials, intermediates, and chemicals utilized to synthesize Tigecycline. To ensure the drug’s optimal efficacy and safety, closely managing and monitoring these impurities is paramount.
Daicel offers a wholesome and integrated Certificate of Analysis (CoA) for Tigecycline impurity standards, encompassing impurities such as 12-oxo-11 hydroxy Tigecycline, 4-Epimer of Tigecycline Hydrochloride, Tigecycline quinone Analog, and Tigecycline Tricyclic Analog. The CoA provides detailed characterization data, including 1H NMR, 13C NMR, IR, MASS, and HPLC purity2. Additionally, we give a detailed 13C-DEPT upon delivery. With advanced technology and expertise, Daicel can synthesize any unknown Tigecycline impurity or degradation product.
References
FAQ's
References
- Sum, Phaik-Eng; Petersen, Peter, Synthesis and structure-activity relationship of novel glycylcycline derivatives leading to the discovery of GAR-936, Bioorganic & Medicinal Chemistry Letters, Volume: 9, Issue: 10, Pages: 1459-1462, 1999
- Li, Chonghua; Sutherland, Christina A.; Nightingale, Charles H.; Nicolau, David P., Quantitation of tigecycline, a novel glycyclcycline, by liquid chromatography, Journal of Chromatography B: Analytical Technologies in the Biomedical and Life Sciences, Volume: 811, Issue: 2, Pages: 225-229, 2004
Frequently Asked Questions
Can Tigecycline impurities affect the drug's bioavailability?
Impurities in Tigecycline can affect the drug's bioavailability and its therapeutic effect.
How are the impurities in Tigecycline controlled during manufacturing?
The control of Tigecycline impurities during the manufacturing process involves monitoring and controlling the manufacturing process, conducting tests at multiple stages of production, and utilizing validated analytical methods.
What are the sources of Tigecycline impurities?
Impurities in Tigecycline may arise from the starting materials, intermediates, degradation products, or residual solvents used in the manufacturing process.
What are the temperature conditions required to store Tigecycline impurities?
Tigecycline impurities are stored at a controlled room temperature between 2-8 °C or as indicated on the Certificate of Analysis (CoA).
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.