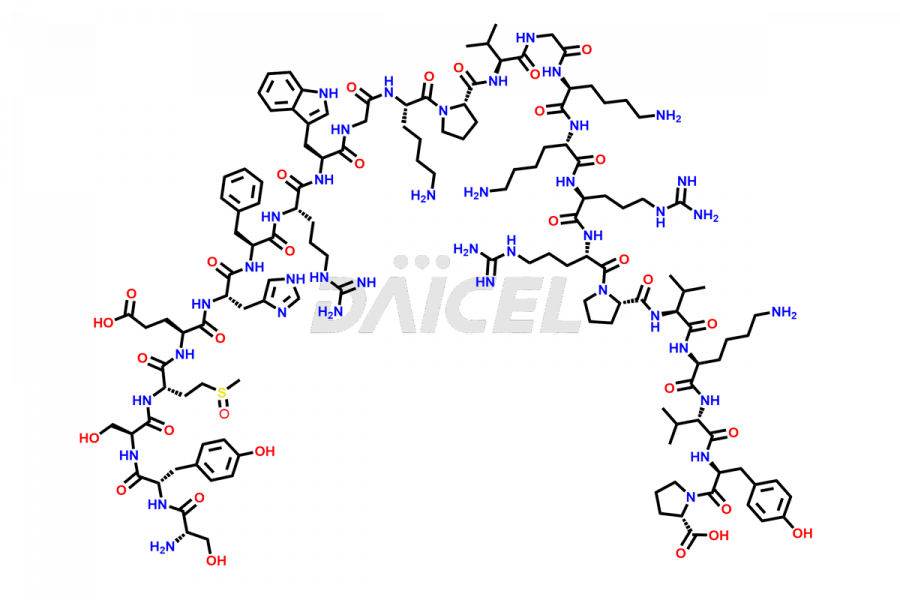
Tetracosactide (Cosyntropin)
General Information
Tetracosactide Impurities and Tetracosactide (Cosyntropin)
Daicel Pharma synthesizes high-quality Tetracosactide (Cosyntropin) impurity, Met(O)-Tetracosactide, essential in evaluating the quality, stability, and biological safety of the active pharmaceutical ingredient, Tetracosactide. Moreover, Daicel offers custom synthesis of Tetracosactide (Cosyntropin) impurities for global delivery.
Cosyntropin, also known as β-Corticotrophin-(1-24)-tetracosapeptide or Tetracosactide [CAS: 16960-16-0], is a synthetic version of the pituitary gland hormone corticotropin (ACTH). Its amino acid sequence is similar to the natural corticotropin’s first 24 amino acids. Cosyntropin is commonly used to evaluate the level of adrenal insufficiency in critically ill patients.
Tetracosactide: Use and Commercial Availability
Tetracosactide (Cosyntropin) is a medication used to help diagnose adrenocortical insufficiency. Due to its rapid effect on the adrenal cortex, Tetracosactide can perform a thirty-minute test on adrenal function. In addition, it has therapeutic use in inflammatory diseases, such as rheumatoid arthritis, ulcerative colitis, Crohn’s disease, etc.
Cosyntropin is available as an intravenous injection. Tetracosactide is available under the brand names, Cortrosyn, Cosyntropin, and Synacthen Depot.
Tetracosactide Structure and Mechanism of Action
The chemical formula for Tetracosactide is C136H210N40O31S, and its molecular weight is approximately 2933.4 g/mol.
Tetracosactide (Cosyntropin) mimics the effects of natural corticotropin but with less immunological activity, as most of the antigenic activity of corticotropin is due to the C-terminal portion of the molecule.
Tetracosactide (Cosyntropin) Impurities and Synthesis
The purity and effectiveness of Tetracosactide, a synthetic peptide used in medical diagnostics, can be compromised by various Tetracosactide (Cosyntropin) impurities that may arise during its synthesis1,2 and storage. Tetracosactide (Cosyntropin) impurities include oxidation products, peptide fragments, and contaminants such as solvents, reagents, and residual starting materials. In addition, synthetic and purification procedures follow GMP while storing the peptide appropriately. Analytical testing methods like HPLC and MS can help identify and measure impurities in Tetracosactide to ensure its quality.
Daicel offers a Certificate of Analysis (CoA) from a cGMP-compliant analytical facility for Tetracosactide (Cosyntropin) impurity standard, Met(O)-Tetracosactide. The CoA includes complete characterization data, such as 1H NMR, 13C NMR, IR, MASS3, and HPLC purity. We also provide 13C-DEPT and CHN on request. We also give a complete characterization report on delivery. Daicel has the technology and expertise to prepare any unknown Tetracosactide (Cosyntropin) impurity or degradation product. The company also provides labeled compounds to quantify the efficacy of generic Tetracosactide. Daicel offers highly pure isotope-labeled standards of Tetracosactide for bioanalytical research and BA/BE studies with isotope data in CoA.
References
- “Tetracosapeptides and Process for their Manufacture”, CIBA LTD, GB1164618 (A), June 17, 1969
- Schwyzer, Robert; Schiller, P. W., “Hormone receptor interactions: synthesis and properties of Nε-dansyllysine21-adrenocorticotropin-(1-24)-tetrakosipeptide”, Helvetica Chimica Acta, Volume: 54, Issue: 3, Pages: 897-904, 1971
- Thevis, Mario; Bredehoeft, Michael; Geyer, Hans; Kamber, Matthias; Delahaut, Philippe; Schaenzer, Wilhelm, “Determination of Synacthen in human plasma using immunoaffinity purification and liquid chromatography/tandem mass spectrometry” Rapid Communications in Mass Spectrometry, Volume: 20, Issue: 23, Pages: 3551-3556, 2006.
Frequently Asked Questions
How is the impurity profile of Tetracosactide determined?
The impurity profile of Tetracosactide involves extensive analytical testing using methods such as HPLC and mass spectrometry. These techniques allow for the identification and quantification of impurities.
How is Tetracosactide purified after synthesis?
Tetracosactide is purified after synthesis using techniques such as HPLC and solid-phase extraction.
What are the stability concerns with Tetracosactide?
Tetracosactide can be prone to oxidation, and degradation over time can lead to decreased potency and increased Tetracosactide (Cosyntropin) impurity levels.
How can the stability of Tetracosactide be improved?
The stability of Tetracosactide improves by storing it at low temperatures, using antioxidant additives, and avoiding exposure to light and moisture.
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.