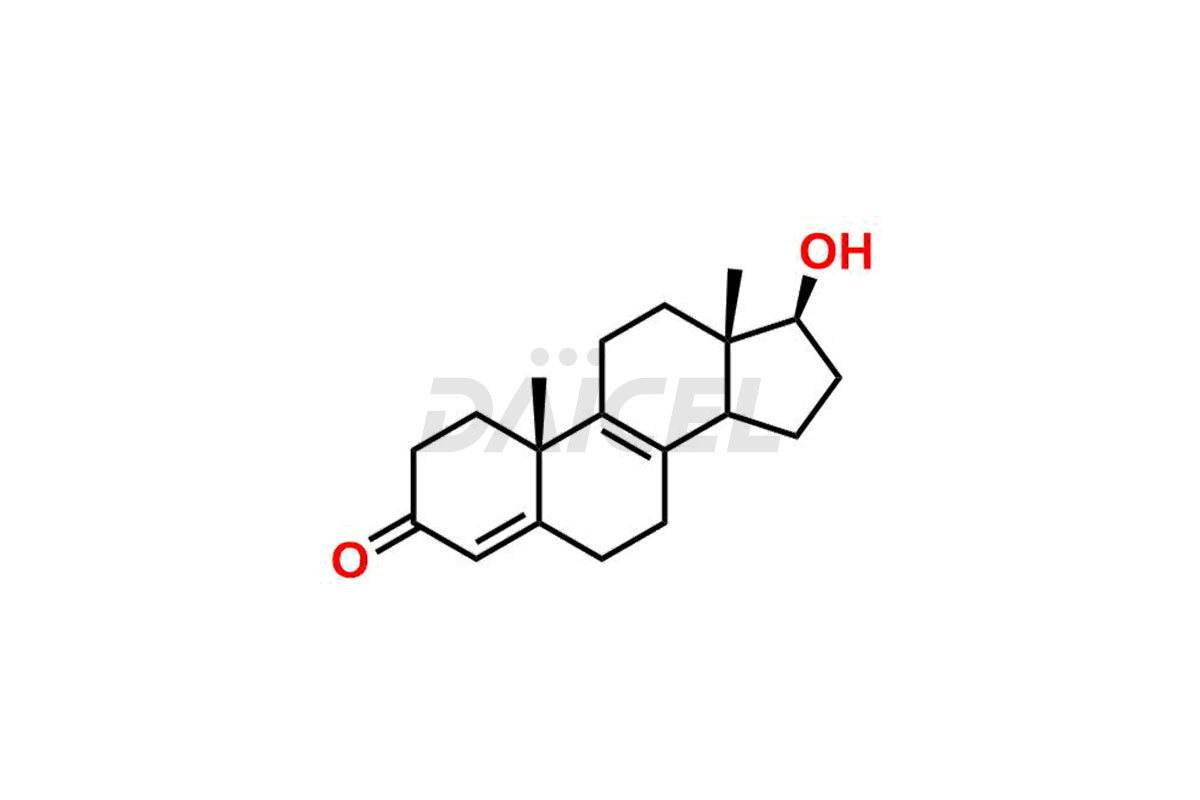
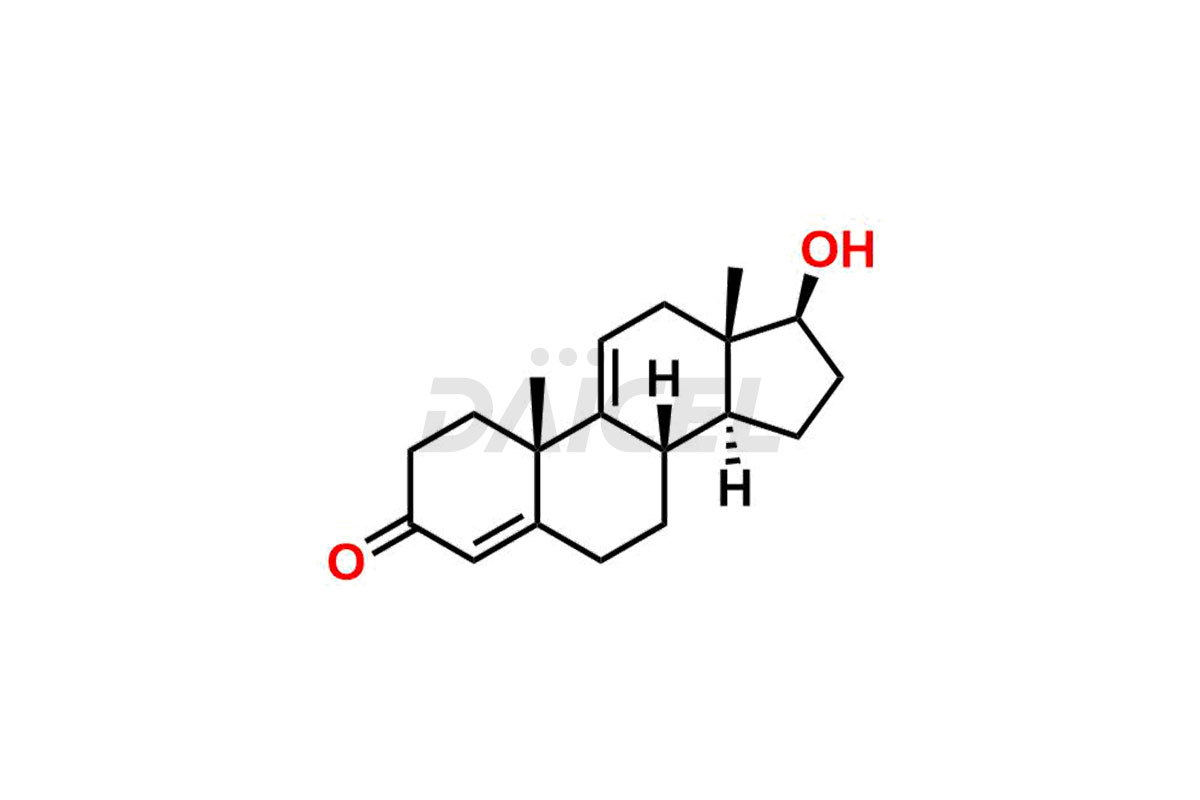
Testosterone
General Information
Testosterone Impurities and Testosterone
Daicel Pharma specializes in providing high-quality Testosterone impurities, Testosterone EP Impurity-K, and Testosterone EP Impurity-L, that ensure the optimal quality, stability, and biological safety of the active pharmaceutical ingredient, Testosterone. These impurities play a crucial role in assessing the overall quality of Testosterone. At Daicel Pharma, we understand that each customer may have unique requirements. That’s why we offer custom-synthesized Testosterone impurities. Our experienced team can tailor the synthesis process to meet the desired specifications, ensuring precise and reliable results.
Testosterone [CAS: 58-22-0] is a vital hormone in developing and maintaining male reproductive tissues, including the testes and prostate. It treats hypoactive sexual desire disorder (HSDD) in women having bilateral oophorectomy or hysterectomy.
Testosterone: Use and Commercial Availability
Testosterone helps in regulating various physiological processes. It plays a significant role in developing secondary sexual characteristics, including the growth of muscle mass, bone density, and body hair. In cases of low testosterone levels, a condition known as hypogonadism, Testosterone acts as hormone replacement therapy (HRT). This therapy aims to restore testosterone levels to their normal range, effectively addressing symptoms such as fatigue, decreased libido, and mood disturbances.
This medicine is available under many brand names – Androderm, Androgel, Natesto, Axiron, Striant, etc. All of them contain the active ingredient Testosterone.
Testosterone Structure and Mechanism of Action 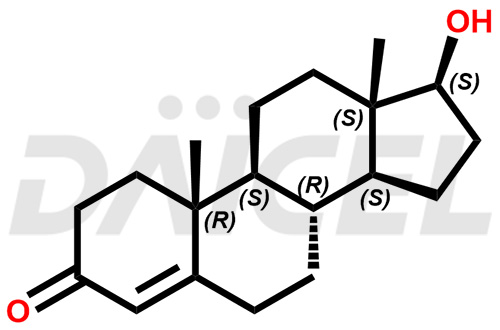
The chemical name of Testosterone is (17β)-17-Hydroxyandrost-4-en-3-one. Its chemical formula is C19H28O2, and its molecular weight is approximately 288.4 g/mol.
Testosterone is responsible for the normal growth and development of the male sex organs and the maintenance of secondary sex characteristics.
Testosterone Impurities and Synthesis
Impurities in Testosterone can vary depending on the synthetic route1 and reaction conditions utilized during its production. It is of utmost importance to closely monitor and regulate the formation of these impurities, as they can impact the drug’s effectiveness.
Daicel Pharma ensures the highest quality by offering a Certificate of Analysis (CoA) from a cGMP-compliant analytical facility for Testosterone impurity standards such as Testosterone EP Impurity-K, and Testosterone EP Impurity-L. The CoA encompasses comprehensive characterization data, such as 1H NMR, 13C NMR, IR, MASS, and HPLC purity2. Upon request, we give additional characterization data such as 13C-DEPT. Daicel Pharma possesses the expertise to synthesize unknown impurities or degradation products of Testosterone.
References
FAQ's
References
- Manufacture of new esters of polynuclear cyclic oxyketones, Soc. pour l'ind. chim. a Bale, GB463163A, March 23, 1937
- Lisboa, Belisario P., Formation and separation of Girard hydrazones on thin-layer chromatography by elatographic techniques, Journal of Chromatography, Volume: 24, Issue: 2, Pages: 475-7, 1966
Frequently Asked Questions
Which solvent helps in the solubility of Testosterone impurities?
Acetonitrile helps to enhance the solubility of Testosterone impurities.
How are Testosterone impurities detected and quantified?
Ultra-performance liquid chromatography (UPLC) method helps detect and quantify impurities in Testosterone.
Can Testosterone impurities affect patient safety?
Yes, impurities in Testosterone can impact patient safety. Depending on their nature and concentration, contaminants can cause adverse effects or reduce the efficacy of the medication.
What are the temperature conditions required to store Testosterone impurities?
Testosterone impurities are stored at a controlled room temperature between 2-8 °C or as indicated on the Certificate of Analysis (CoA).
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.