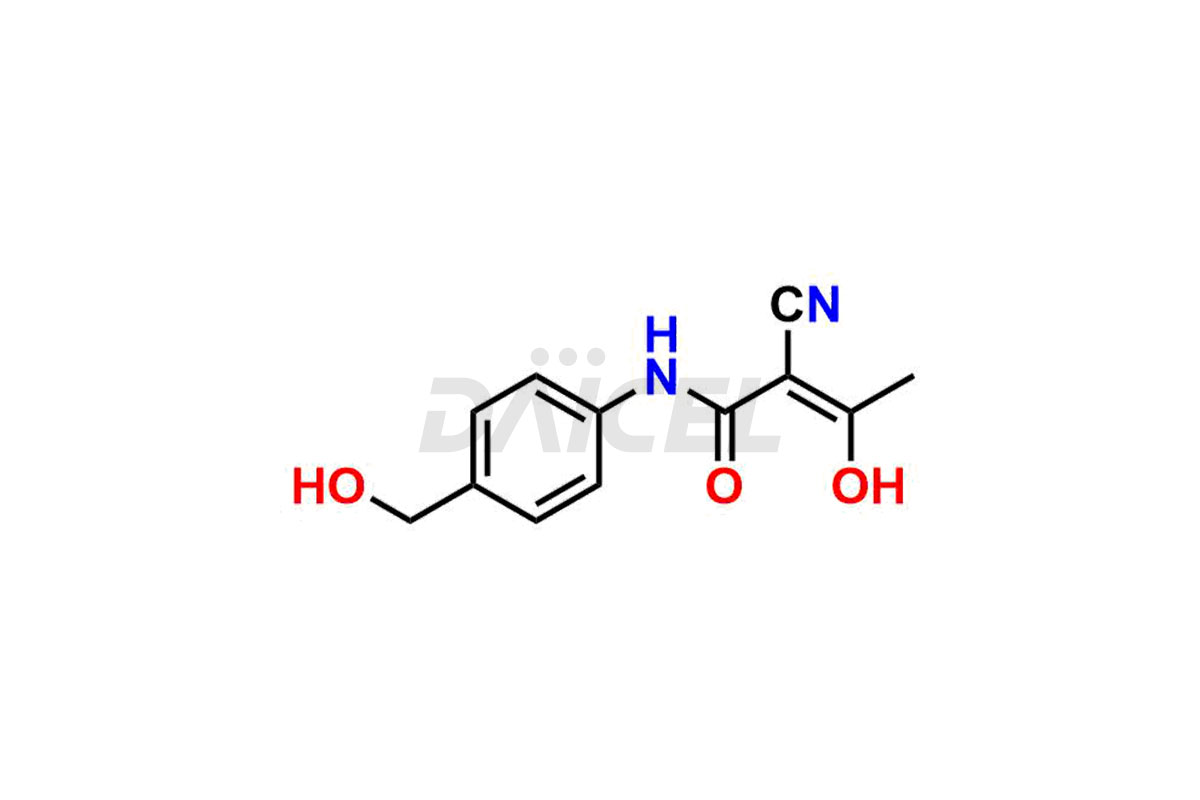
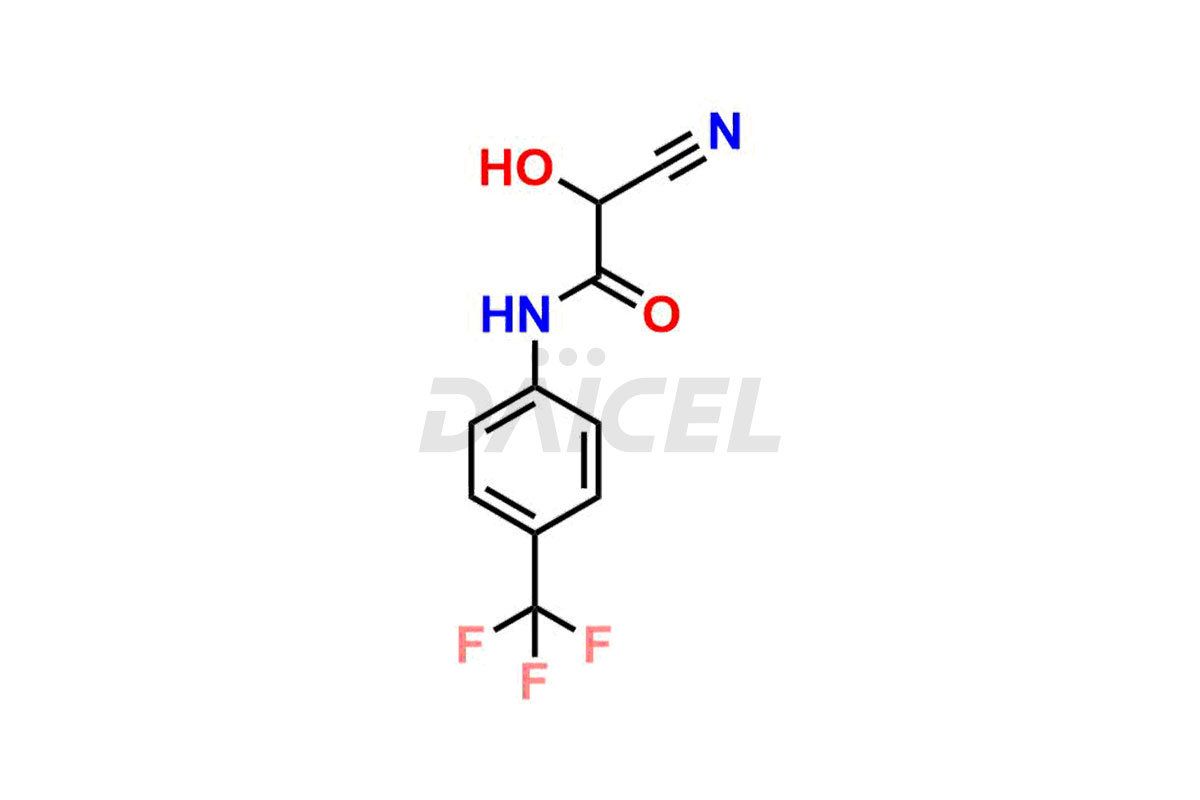
Teriflunomide
General Information
Teriflunomide Impurities and Teriflunomide
Daicel Pharma offers optimal Teriflunomide impurities such as 3-Trifluoromethyl Teriflunomide, Pentanamide compound (teriflunomide), Teriflunomide imp-B and Teriflunomide Impurity They help analyze the quality, stability, and biological safety of the active pharmaceutical ingredient, Teriflunomide. Daicel Pharma can synthesize Teriflunomide impurities and ensure global delivery to customer requirements.
Teriflunomide [CAS: 163451-81-8] is a novel disease-modifying agent indicated for treating multiple sclerosis (MS). It is a tyrosine kinase inhibitor and a non-steroidal anti-inflammatory drug.
Teriflunomide: Use and Commercial Availability
Teriflunomide manages relapsing forms of multiple sclerosis (MS) and rheumatoid arthritis. It is an active metabolite of Leflunomide, an immunosuppressant.
This medicine is available under Aubagio, which contains the active ingredient Teriflunomide.
Teriflunomide Structure and Mechanism of Action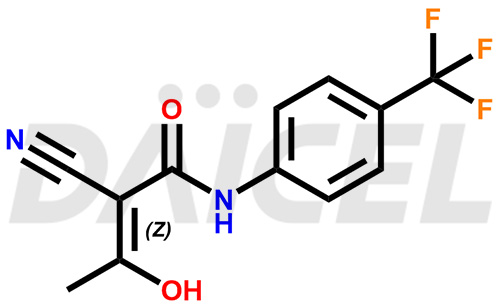
The chemical name of Teriflunomide is (2Z)-2-Cyano-3-hydroxy-N-[4-(trifluoromethyl)phenyl]-2-butenamide. Its chemical formula is C12H9F3N2O2, and its molecular weight is approximately 270.21 g/mol.
Teriflunomide may reduce the number of activated lymphocytes in the CNS, but its exact mechanism of action is unknown.
Teriflunomide Impurities and Synthesis
Teriflunomide impurities form during synthesis1 and vary depending on the synthetic route and the reaction conditions. Vigilant monitoring and regulation of impurity formation are essential, as these impurities can affect the drug’s efficacy.
Daicel Pharma offers a Certificate of Analysis (CoA) from a cGMP-compliant analytical facility for Teriflunomide impurity standards like 3-Trifluoromethyl Teriflunomide, Pentanamide compound (teriflunomide), Teriflunomide imp-B and Teriflunomide Impurity. The CoA includes complete characterization data, such as 1H NMR, 13C NMR, IR, MASS, and HPLC purity2. We give additional characterization data, including 13C-DEPT, on request. Daicel Pharma can synthesize unknown impurities or degradation products of Teriflunomide.
References
FAQ's
References
- Patterson, John W.; Cheung, Paul S.; Ernest, Michael J., 3-Carboxy-5-methyl-N-[4-(trifluoromethyl)phenyl]-4-isoxazolecarboxamide, new prodrug for the antiarthritic agent 2-cyano-3-hydroxy-N-[4-(trifluoromethyl)phenyl]-2-butenamide, Journal of Medicinal Chemistry, Volume: 35, Issue: 3, Pages: 507-10, 1992
- Sobhani, Kimia; Garrett, Danette A.; Liu, Dong-Pei; Rainey, Petrie M., A rapid and simple high-performance liquid chromatography assay for the leflunomide metabolite, teriflunomide (A77 1726), in renal transplant recipients, American Journal of Clinical Pathology, Volume: 133, Issue: 3, Pages: 454-457, 2010
Frequently Asked Questions
How are Teriflunomide impurities detected and quantified?
Analytical methods like LC-MS/MS can detect and quantify impurities in Teriflunomide.
Can Teriflunomide impurities affect patient safety?
Impurities in Teriflunomide can impact patient safety. Depending on their nature and concentration, contaminants can cause adverse effects or reduce the efficacy of the medication.
Which solvent helps in the solubility of Teriflunomide impurities?
Methanol helps to enhance the solubility of Teriflunomide impurities
What are the temperature conditions required to store Teriflunomide impurities?
Teriflunomide impurities are stored at a controlled room temperature between 2-8 ⁰C or as indicated on the Certificate of Analysis (CoA).
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.