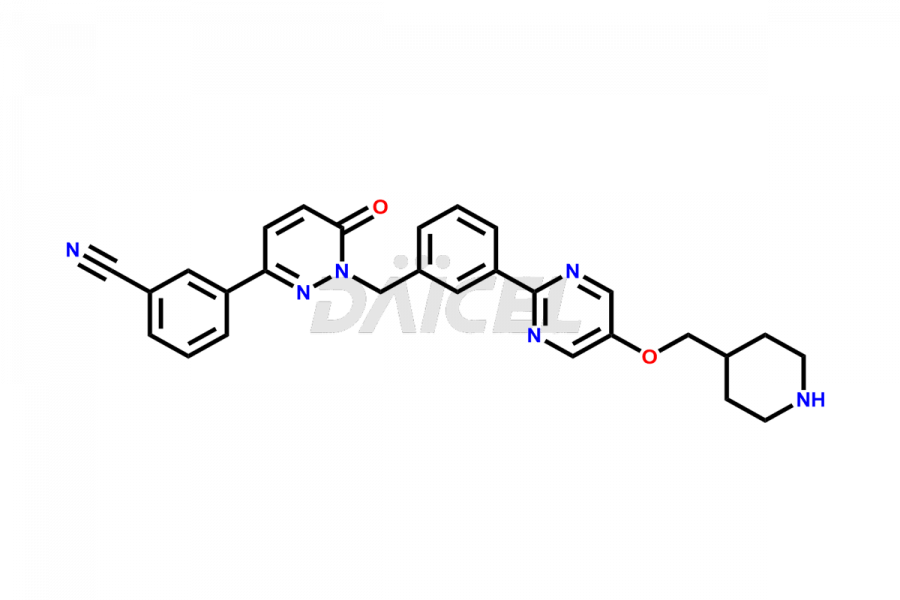
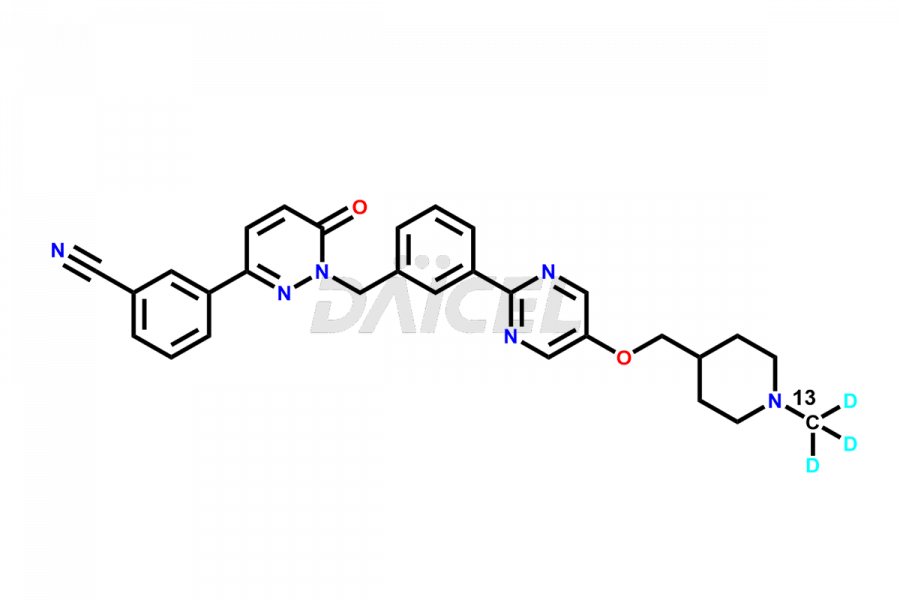
Tepotinib
General Information
Tepotinib Impurities and Tepotinib
Daicel Pharma offers optimal Tepotinib impurities, such as Desmethyl Tepotinib. The active pharmaceutical ingredient Tepotinib ‘s quality, stability, and biological safety depend on the impurities. Daicel Pharma can also synthesize Tepotinib impurities according to the customers’ specific needs and deliver them worldwide.
Tepotinib [CAS: 1100598-32-0] is a MET tyrosine kinase inhibitor that treats many MET-overexpressing solid tumors and non-small cell lung cancer (NSCLC).
Tepotinib: Use and Commercial Availability
Tepotinib treats advanced or metastatic non-small cell lung cancer (NSCLC). It targets the growth and spread of cancer cells by selectively inhibiting the MET receptor tyrosine kinase. It aims to slow down the progression of NSCLC by inhibiting MET activity, offering potential benefits to eligible patients.
This medicine is available under Tepmetko, which contains the active ingredient Tepotinib.
Tepotinib Structure and Mechanism of Action 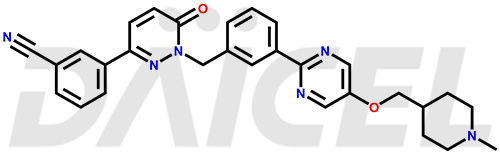
The chemical name of Tepotinib is 3-[1,6-Dihydro-1-[[3-[5-[(1-methyl-4-piperidinyl)methoxy]-2-pyrimidinyl]phenyl]methyl]-6-oxo-3-pyridazinyl]benzonitrile. Its chemical formula is C29H28N6O2, and its molecular weight is approximately 492.6 g/mol.
Tepotinib inhibits hepatocyte growth factor (HGF)-dependent and -independent MET phosphorylation, melatonin 2 and imidazoline 1 receptors, and MET-dependent downstream signaling pathways.
Tepotinib Impurities and Synthesis
Tepotinib impurities generate during synthesis1 and vary depending on the synthetic route and the reaction conditions used. Vigilant monitoring and regulation of impurity formation are essential, as these impurities can affect the drug’s efficacy.
Daicel offers a Certificate of Analysis (CoA) from a cGMP-compliant analytical facility for the Tepotinib impurity standard, Desmethyl Tepotinib. The CoA includes complete characterization data, such as 1H NMR, 13C NMR, IR, MASS, and HPLC purity2. We give additional characterization data, including 13C-DEPT, on request. Daicel Pharma can synthesize unknown impurities or degradation products of Tepotinib and supply labeled compounds for assessing the effectiveness of generic versions. Furthermore, we also provide highly pure Tepotinib 13CD3, deuterium-labeled standards of Tepotinib, which is crucial for bioanalytical research and BA/BE (Bioavailability/Bioequivalence) studies.
References
FAQ's
References
Frequently Asked Questions
How are Tepotinib impurities detected and quantified?
Analytical methods, such as high-performance liquid chromatography (HPLC), are commonly used to detect and quantify impurities in Tepotinib.
Can Tepotinib impurities affect patient safety?
Yes, impurities in Tepotinib can impact patient safety. Depending on their nature and concentration, contaminants can cause adverse effects or reduce the efficacy of the medication.
Why is it essential to synthesize Tepotinib impurities?
It is essential to synthesize impurities of Tepotinib to accurately identify, characterize, and establish their permissible limits within the drug product.
What are the temperature conditions required to store Tepotinib impurities?
Tepotinib impurities are stored at a controlled room temperature between 2-8 °C or as indicated on the Certificate of Analysis (CoA).
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.