Teneligliptin
General Information
Teneligliptin Impurities and Teneligliptin
Daicel Pharma offers a diverse range of exclusive Teneligliptin impurities, such as
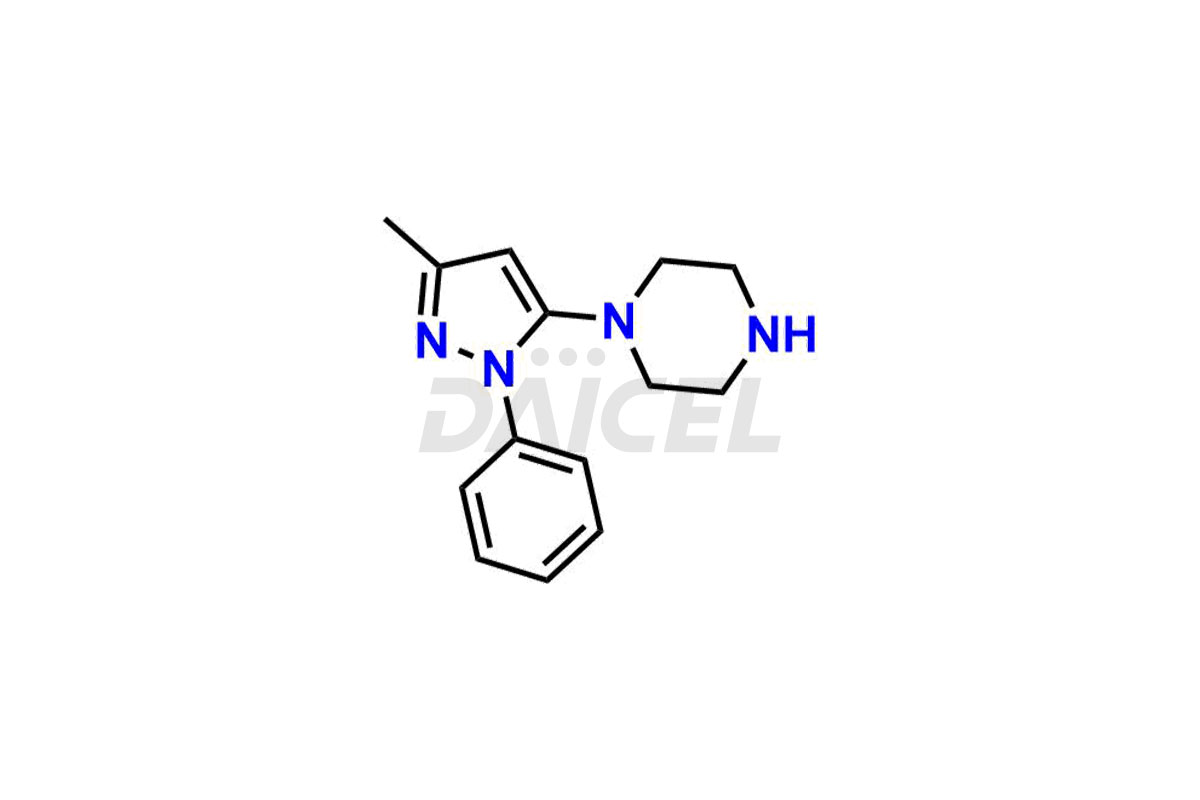
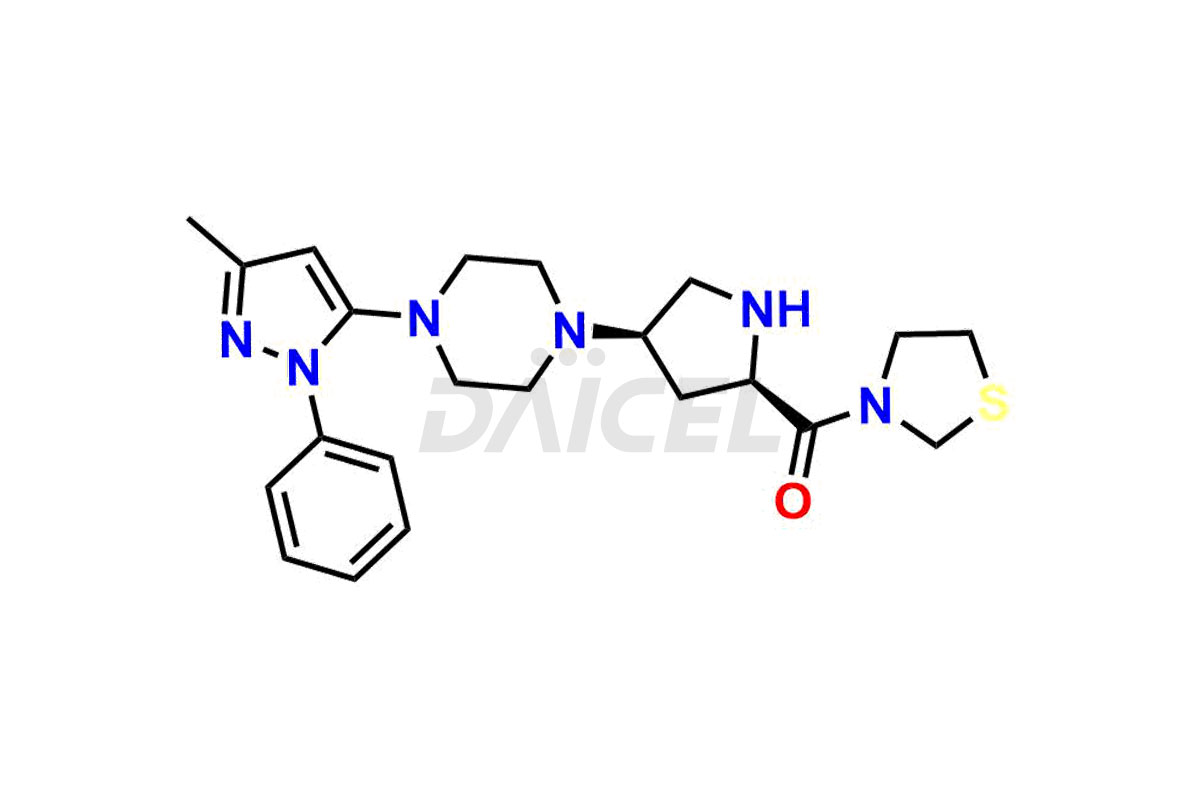
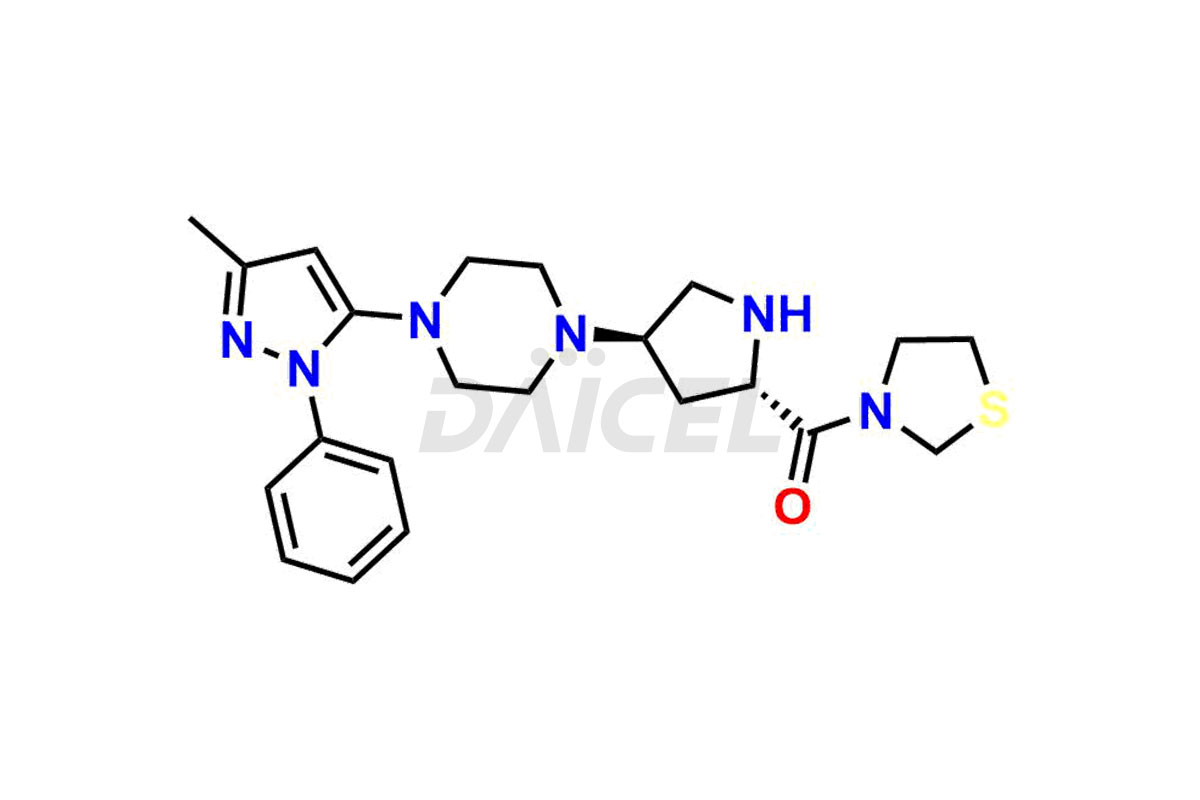
1-(3-methyl-1-phenyl-1H-pyrazol-5-yl)piperazine, Teneligliptin (2R,4R)-Isomer, Teneligliptin (2R,4S)-isomer and Teneligliptin (2S,4R)-isomer. These impurities are significant in assessing the quality, stability, and biological safety of the active pharmaceutical ingredient Teneligliptin. Moreover, Daicel Pharma offers custom synthesis of Teneligliptin impurities and delivers them globally to meet the specific requirements of the clientele.
Teneligliptin [CAS: 760937-92-6] is an orally bioavailable, long-acting pyrrolidine-based dipeptidyl peptidase 4 (DPP-4) inhibitor exhibiting hypoglycemic activity. It manages type 2 diabetes mellitus (T2DM).
Teneligliptin: Use and Commercial Availability
Teneligliptin treats type 2 diabetes mellitus. As an inhibitor of dipeptidyl peptidase 4 (DPP-4), this drug helps regulate blood glucose levels by increasing the activity of incretin hormones, such as GLP-1 (glucagon-like peptide-1). By inhibiting the degradation of these hormones, Teneligliptin promotes insulin secretion and reduces glucagon release, leading to improved glycemic control.
Teneligliptin is available under Teneria, Tenepure, Teneza, etc., containing the active ingredient Teneligliptin.
Teneligliptin Structure and Mechanism of Action 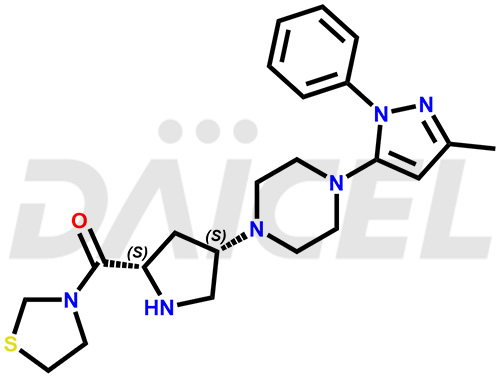
The chemical name of Teneligliptin is [(2S,4S)-4-[4-(3-Methyl-1-phenyl-1H-pyrazol-5-yl)-1-piperazinyl]-2-pyrrolidinyl]-3-thiazolidinylmethanone. Its chemical formula is C22H30N6OS, and its molecular weight is approximately 426.6 g/mol.
The mechanism of action of Teneligliptin is not known.
Teneligliptin Impurities and Synthesis
During the synthesis1 of Teneligliptin, various impurities like related substances, degradation products, and residual solvents can generate. It is vital to carefully monitor and control them to ensure the drug’s safety, efficacy, and overall quality.
Daicel Pharma offers a comprehensive Certificate of Analysis (CoA) for Teneligliptin impurity standards, 1-(3-methyl-1-phenyl-1H-pyrazol-5-yl)piperazine, Teneligliptin (2R,4R)-Isomer, Teneligliptin (2R,4S)-isomer and Teneligliptin (2S,4R)-isomer. They are manufactured in compliance with current Good Manufacturing Practices (cGMP). The CoA includes complete characterization data, such as 1H NMR, 13C NMR, IR, MASS, and HPLC purity2. We give additional characterization data, including 13C-DEPT, on request. Daicel Pharma can generate unknown impurities or degradation products of Teneligliptin.
References
FAQ's
References
- Yoshida, Tomohiro; Sakashita, Hiroshi; Ueda, Naoko; Kirihara, Shinji; Uemori, Satoru; Tsutsumiuchi, Reiko; Akahoshi, Fumihiko, Salt of proline derivative, solvate thereof, and production method thereof, Mitsubishi Pharma Corporation, Japan, US8003790B2, August 23, 2011
- Chunduri, Raja Haranadha Babu; Dannana, Gowri Sankar, Development and validation of LC-MS/MS method for quantification of teneligliptin in human plasma and its application to a pharmacokinetic study, World Journal of Pharmacy and Pharmaceutical Sciences, Volume: 5, Issue: 5, Pages: 838-850, 2016
Frequently Asked Questions
How are Teneligliptin impurities detected and quantified?
RP-HPLC method can detect impurities effectively in Teneligliptin.
Can Teneligliptin impurities affect patient safety?
The presence of impurities in Teneligliptin can have implications for patient safety. Different types and levels of impurities can cause adverse effects or compromise the efficacy of the medication.
Which solvents help in the analysis of Teneligliptin impurities?
Methanol is used to achieve optimal solubility and separation of Teneligliptin impurities.
What are the temperature conditions required to store Teneligliptin impurities?
Teneligliptin impurities are stored at a controlled room temperature between 2-8 °C or as indicated on the Certificate of Analysis (CoA).
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.