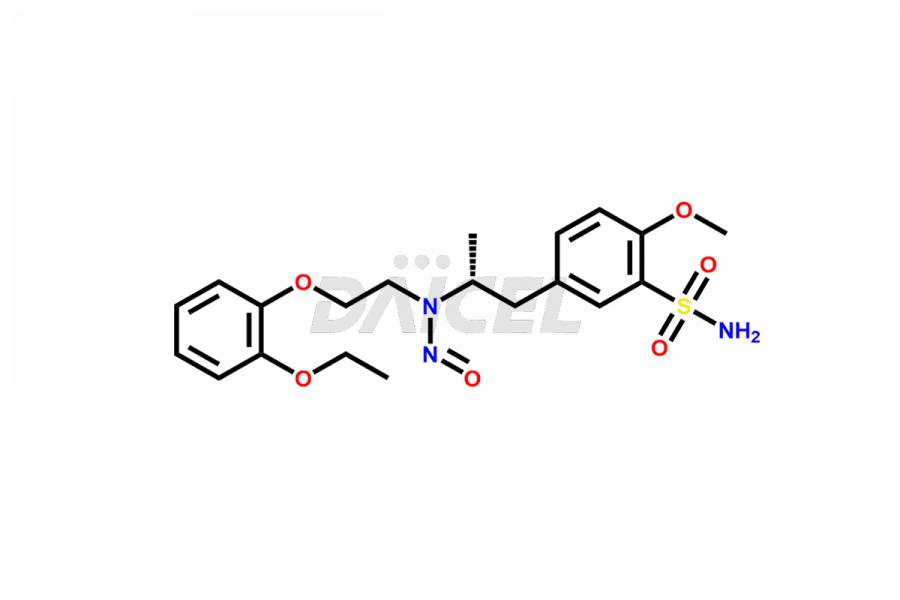
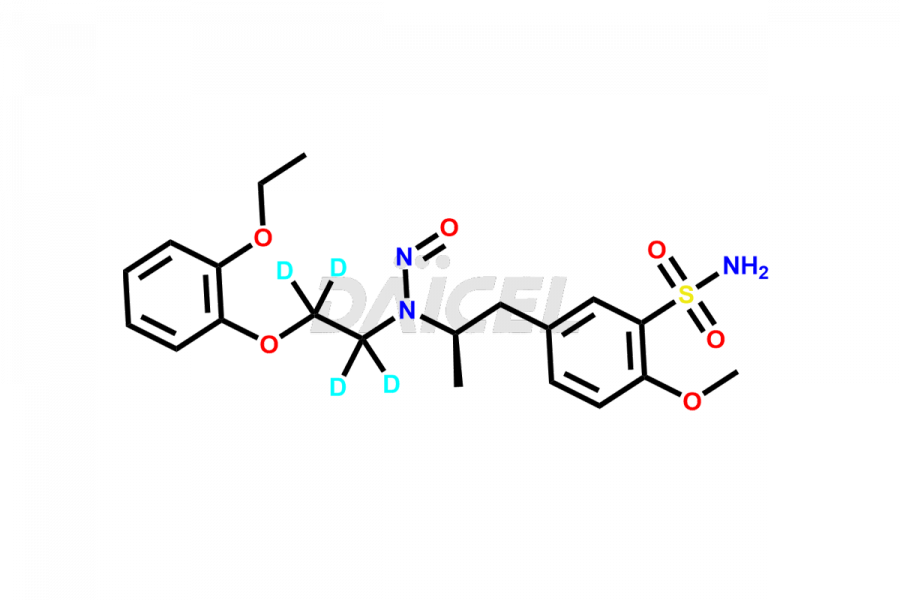
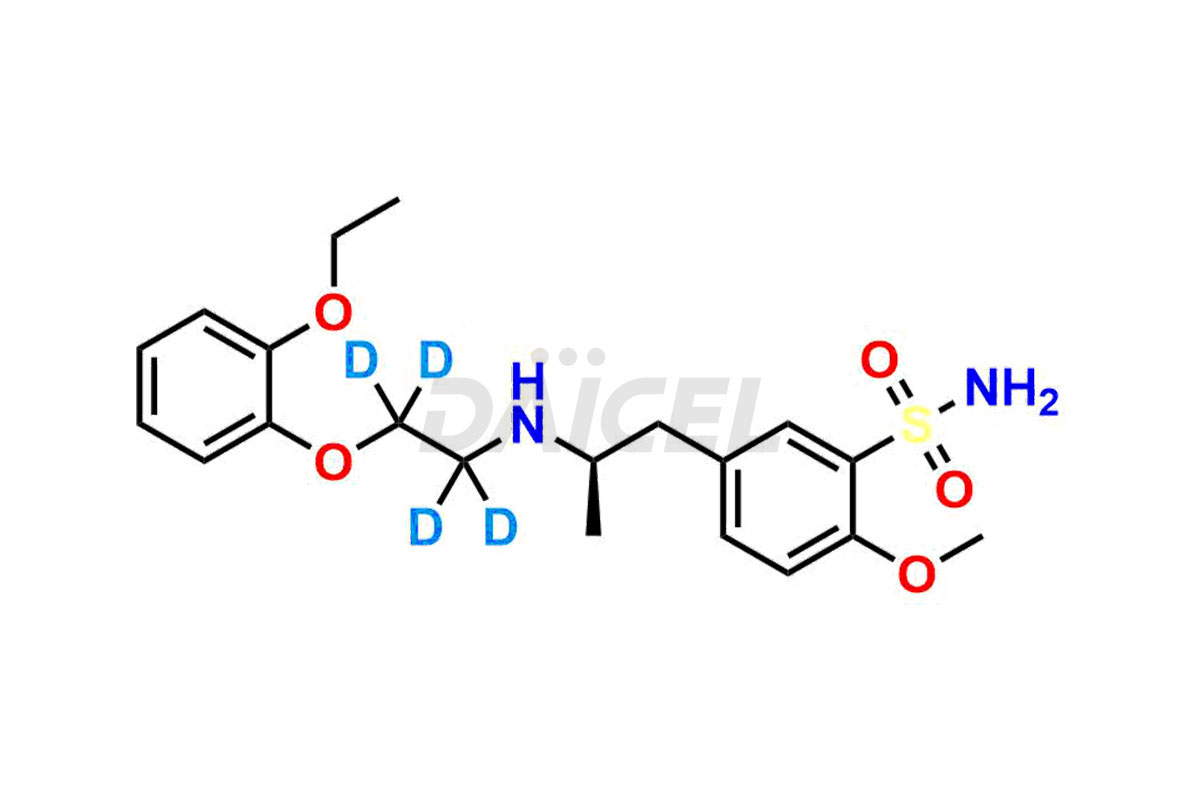
Tamsulosin
General Information
Tamsulosin Impurities and Tamsulosin
Daicel Pharma provides a wide selection of high-quality Tamsulosin impurity standards, including N-Nitroso Tamsulosin Impurity. These impurities are vital in evaluating the active pharmaceutical ingredient Tamsulosin’s quality, stability, and biological safety. Moreover, Daicel Pharma can synthesize Tamsulosin impurities based on specific customer requirements, ensuring reliable delivery worldwide.
Tamsulosin [CAS: 106133-20-4] treats the signs and symptoms of benign prostatic hyperplasia (BPH). It belongs to a class of alpha-1 adrenergic blockers. This drug is for conditions such as ureteral stones, prostatitis, and female voiding dysfunction.
Tamsulosin: Use and Commercial Availability
Tamsulosin, a specific antagonist of alpha-1A and alpha-1B adrenoceptors, primarily acts on the prostate and bladder found in these receptors. It manages symptoms associated with benign prostatic hypertrophy. By blocking these receptors, it induces smooth muscle relaxation in the prostate and detrusor muscles of the bladder, resulting in improved urinary flow.
Tamsulosin is available under Flomax, which contains the active ingredient Tamsulosin.
Tamsulosin Structure and Mechanism of Action 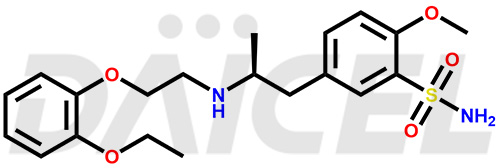
The chemical name of Tamsulosin is 5-[(2R)-2-[[2-(2-Ethoxyphenoxy)ethyl]amino]propyl]-2-methoxybenzenesulfonamide. Its chemical formula is C20H28N2O5S, and its molecular weight is approximately 408.5 g/mol
Tamsulosin blocks alpha-1D adrenoceptors and relaxes the detrusor muscles of the bladder.
Tamsulosin Impurities and Synthesis
During the synthesis1 and storage of Tamsulosin, various impurities such as related substances, degradation products, and residual solvents can form. It is crucial to diligently monitor and control these impurities to ensure the medication’s safety, effectiveness, and overall quality.
Daicel Pharma provides a comprehensive Certificate of Analysis (CoA) for Tamsulosin impurity standards, such as N-Nitroso Tamsulosin Impurity. These impurities are produced strictly with current Good Manufacturing Practices (cGMP). The CoA comprises detailed characterization data, such as 1H NMR, 13C NMR, IR, MASS, and HPLC purity2, offering a thorough understanding of the impurity profile. Upon request, Daicel can also supply 13C-DEPT data for further characterization.
Additionally, Daicel Pharma possesses the technical expertise to synthesize any unknown impurities or degradation products of Tamsulosin. We also offer labeled compounds that help assess the efficacy of generic versions of Tamsulosin. For bioanalytical research and BA/BE studies, we also provide deuterium-labeled Tamsulosin standards such as Tamsulosin D4 and N-Nitroso-Tamsulosin d4 Impurity (Mixture of isomers).
References
FAQ's
References
- Niigata, Kunihiro; Fujikura, Takashi, Sulfamoyl-substituted phenethylamine derivatives, their preparation, and pharmaceutical compositions, containing them, Yamanouchi Pharmaceutical Co., Ltd., US4731478A, March 15, 1988
- Matsushima, Hiroshi; Takanuki, Ken-ichi; Kamimura, Hidetaka; Watanabe, Takashi; Higuchi, Saburo, Highly sensitive method for the determination of tamsulosin hydrochloride in human plasma dialyzate, plasma and urine by high-performance liquid chromatography-electrospray tandem mass spectrometry, Journal of Chromatography B: Biomedical Sciences and Applications, Volume: 695, Issue: 2, Pages: 317-327, 1997
Frequently Asked Questions
How are Tamsulosin impurities detected and quantified?
Impurities in Tamsulosin can be detected using analytical techniques like High-Performance Liquid Chromatography (HPLC).
Can Tamsulosin impurities affect patient safety?
The presence of impurities in Tamsulosin can affect patient safety. The type and level of these impurities can lead to adverse effects or diminish the effectiveness of the medication.
Which solvents help in the analysis of Tamsulosin impurities?
Acetonitrile is used to achieve optimal solubility and separation of Tamsulosin impurities.
5. What are the temperature conditions required to store Tamsulosin impurities?
Tamsulosin impurities are stored at a controlled room temperature between 2-8 °C or as indicated on the Certificate of Analysis (CoA).
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.