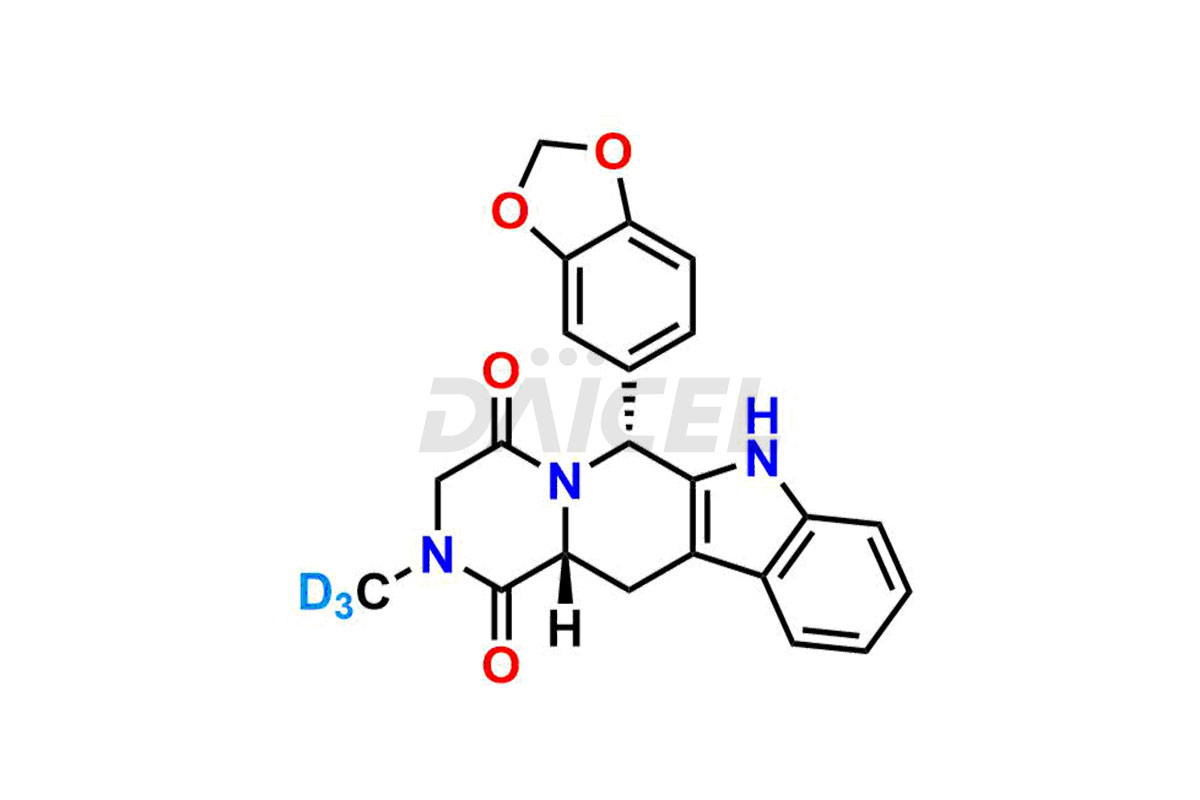
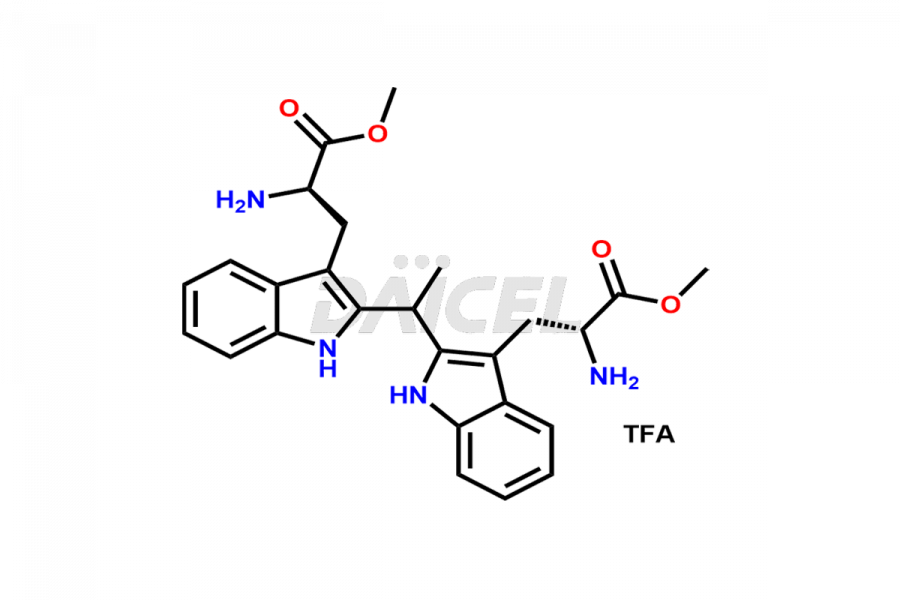
Tadalafil
General Information
Tadalafil Impurities and Tadalafil
Daicel Pharma offers a comprehensive range of Tadalafil impurities, such as Tadalafil bis(2-aminopropanoate) dimer impurity. The presence of impurities plays a crucial role in assessing the quality, stability, and biological safety of Tadalafil, an active pharmaceutical ingredient. Daicel Pharma can synthesize Tadalafil impurities based on specific customer requirements, ensuring precise customization and worldwide delivery.
Tadalafil [CAS: 171596-29-5] is utilized for the management of erectile dysfunction (ED) and can be used alone or in combination with finasteride for the treatment of benign prostatic hyperplasia (BPH). Additionally, it treats pulmonary arterial hypertension (PAH), either as a standalone therapy or in combination with Macitentan or other endothelin-1 antagonists.
Tadalafil: Use and Commercial Availability
Tadalafil treats erectile dysfunction (ED) by increasing sexual stimulation dependant smooth muscle relaxation in the penis. It helps to manage benign prostatic hyperplasia (BPH) by reducing the size of the prostate. It is also effective in treating pulmonary arterial hypertension (PAH) and helps to relax and widen blood vessels, improving blood flow and alleviating symptoms.
Tadalafil is available under many brand names. Some of them are Adcirca, Alyq, Cialis, and Tadliq. All of these contain the active ingredient, Tadalafil.
Tadalafil Structure and Mechanism of Action 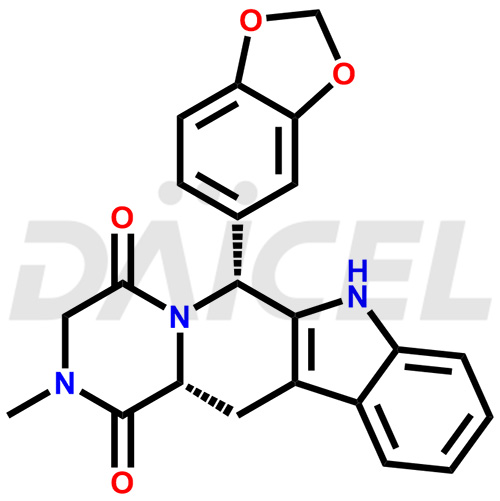
The chemical name of Tadalafil is (6R,12aR)-6-(1,3-Benzodioxol-5-yl)-2,3,6,7,12,12a-hexahydro-2-methylpyrazino[1′,2′:1,6]pyrido[3,4-b]indole-1,4-dione. Its chemical formula is C22H19N3O4, and its molecular weight is approximately 389.4 g/mol.
Tadalafil inhibits phosphodiesterase type 5 (PDE5), which enhances erectile function by increasing the amount of Cyclic GMP.
Tadalafil Impurities and Synthesis
During the synthesis1 and storage of Tadalafil, impurities can arise, including related substances, degradation products, and residual solvents. It is essential to carefully monitor and control these impurities to ensure the medication’s safety, efficacy, and overall quality.
Daicel Pharma offers a comprehensive Certificate of Analysis (CoA) for Tadalafil impurity standards, like Tadalafil bis(2-aminopropanoate) dimer impurity. Its synthesis complies with current Good Manufacturing Practices (cGMP). The CoA includes detailed characterization data, such as 1H NMR, 13C NMR, IR, MASS, and HPLC purity2, providing a thorough understanding of the impurity profile. Upon request, we can also give 13C-DEPT data.
Furthermore, Daicel Pharma possesses the technical expertise to synthesize any unknown impurities or degradation products of Tadalafil. We also offer labeled compounds to evaluate the effectiveness of generic versions of Tadalafil. For bioanalytical research and Bioavailability/Bioequivalence (BA/BE) studies, Daicel supplies Tadalafil – D3, a highly pure deuterium-labeled standard of Tadalafil.
References
FAQ's
Frequently Asked Questions
How are Tadalafil impurities detected and quantified?
Analytical Methods such as High-Performance Liquid Chromatography (RP-HPLC) can detect impurities in Tadalafil.
Can Tadalafil impurities affect patient safety?
Impurities in Tadalafil can impact patient safety. Depending on their nature and concentration, contaminants can cause adverse effects or reduce the efficacy of the medication.
Which solvents help in the analysis of Tadalafil impurities?
Methanol is employed to attain the ideal solubility and differentiation of impurities in Tadalafil.
What are the temperature conditions required to store Tadalafil impurities?
Tadalafil impurities are stored at a controlled room temperature between 2-8 ⁰C or as indicated on the Certificate of Analysis (CoA).
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.