LOAD MORE
You're viewed 9 of 10 products
Daicel Pharma synthesizes high-quality Sumatriptan impurities like Sumatriptan EP Impurity E, Sumatriptan Impurity 2, Sumatriptan Impurity BP Imp-1/Sumatriptan pyrroloindolium analog, Sumatriptan Impurity-A, Sumatriptan impurity-F, Sumatriptan N-oxide, and Sumatriptan Succinate Impurity, which are crucial in the analysis of the quality, stability, and biological safety of the active pharmaceutical ingredient Sumatriptan. Moreover, Daicel Pharma offers custom synthesis of Sumatriptan impurities and delivers them globally.
Sumatriptan [CAS: 103628-46-2] is an FDA-approved medicine treating migraine attacks. It treats cluster headache episodes through subcutaneous administration. It is a sulfonamide triptan that causes vasoconstriction of cranial and basilar arteries.
Sumatriptan is effective and well-tolerated medicine for migraines, whether administered intranasally, subcutaneously, or orally. Its use helps to relieve photophobia, nausea, headache, and functional disability. Sumatriptan is available under brand names such as Alsuma, Imitrex, Onzetra Xsail, Sumavel Dosepro, Tosymra, Zecuity, and Zembrace Symtouch.
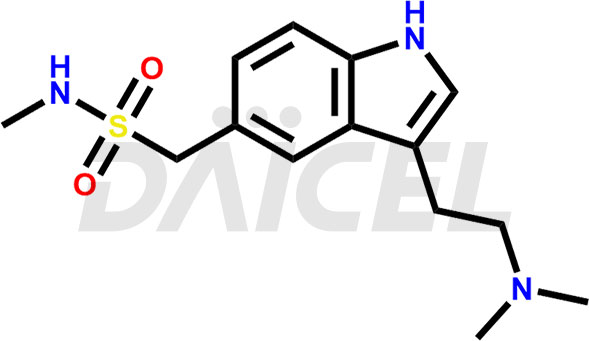
The chemical name of Sumatriptan is 3-[2-(Dimethylamino)ethyl]-N-methyl-1H-indole-5-methanesulfonamide. Its chemical formula is C14H21N3O2S, and its molecular weight is approximately 295.40 g/mol.
Sumatriptan is a vascular-5HT1 receptor subtype agonist that causes vasoconstriction. It acts on human basilar arteries and helps in relieving migraine headaches.
Sumatriptan forms impurities during its synthetic1 process. These impurities are influenced by reaction conditions, starting materials, and purification techniques. Rigorous quality control measures monitor and remove impurities during synthesis to ensure the safety and efficacy of the final product.
Daicel provides a Certificate of Analysis (CoA) for Sumatriptan impurity standards, including Sumatriptan EP Impurity E, Sumatriptan Impurity 2, Sumatriptan Impurity BP Imp-1/Sumatriptan pyrroloindolium analog, Sumatriptan Impurity-A, Sumatriptan impurity-F, Sumatriptan N-oxide, and Sumatriptan Succinate Impurity. The CoA is issued from a cGMP-compliant analytical facility and contains complete characterization data, such as 1H NMR, 13C NMR, IR, MASS, and HPLC purity2,3. Additional characterization data, such as 13C-DEPT and CHN, can be provided upon request. Daicel can also prepare any unknown Sumatriptan impurity or degradation product and offer labeled compounds to quantify the efficacy of Sumatriptan. Daicel offers Sumatriptan D6 Format Salt, a deuterium-labeled Sumatriptan compound used in bio-analytical research such as BA/BE studies. We give a complete characterization report on delivery.
The impurities in Sumatriptan are detected and quantified using various analytical techniques such as Reverse Phase High-Performance Liquid Chromatography (RP-HPLC).
Controlling the presence of impurities in Sumatriptan is crucial for ensuring that the drug is of high quality, safe, and effective. Further, they may harm various aspects of the drug's performance, such as its potency, stability, and overall shelf life.
Water or DMSO is the solvent used in analyzing many impurities in Sumatriptan.
Sumatriptan impurities are stored at a controlled room temperature between 2-8 ⁰C or as indicated on the Certificate of Analysis (CoA).
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.