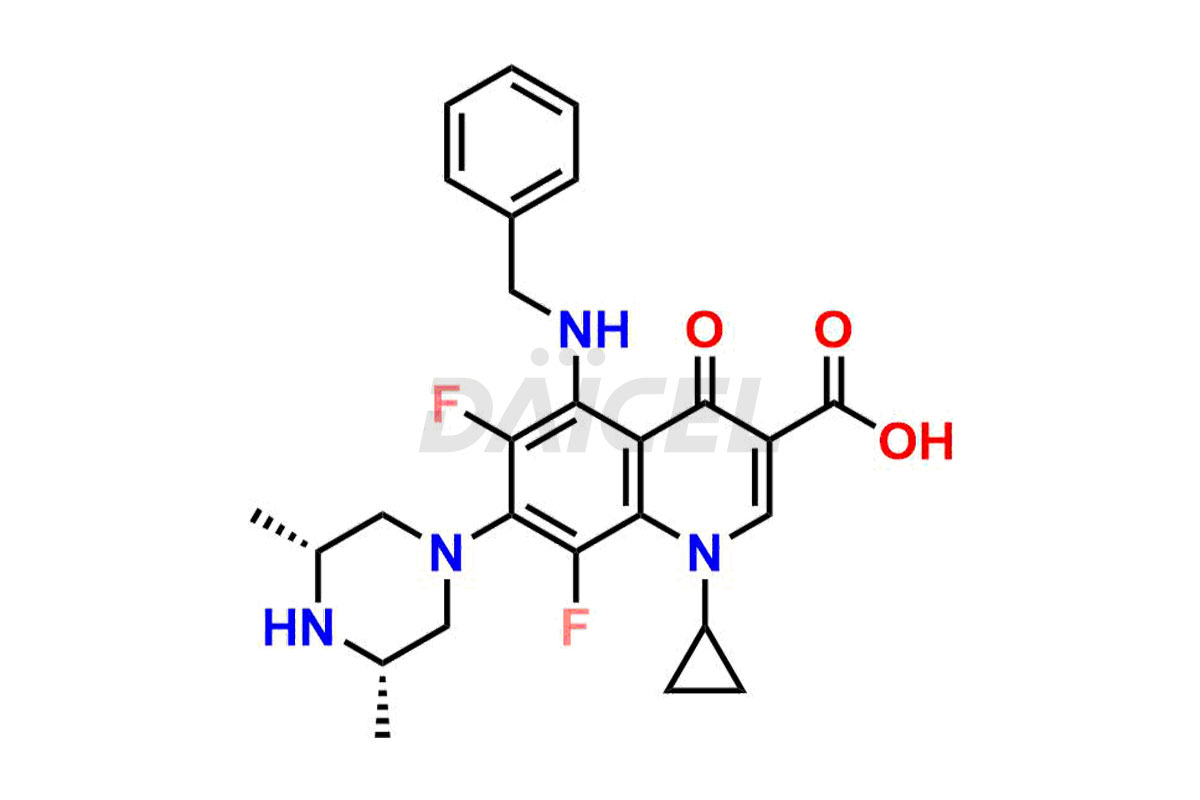
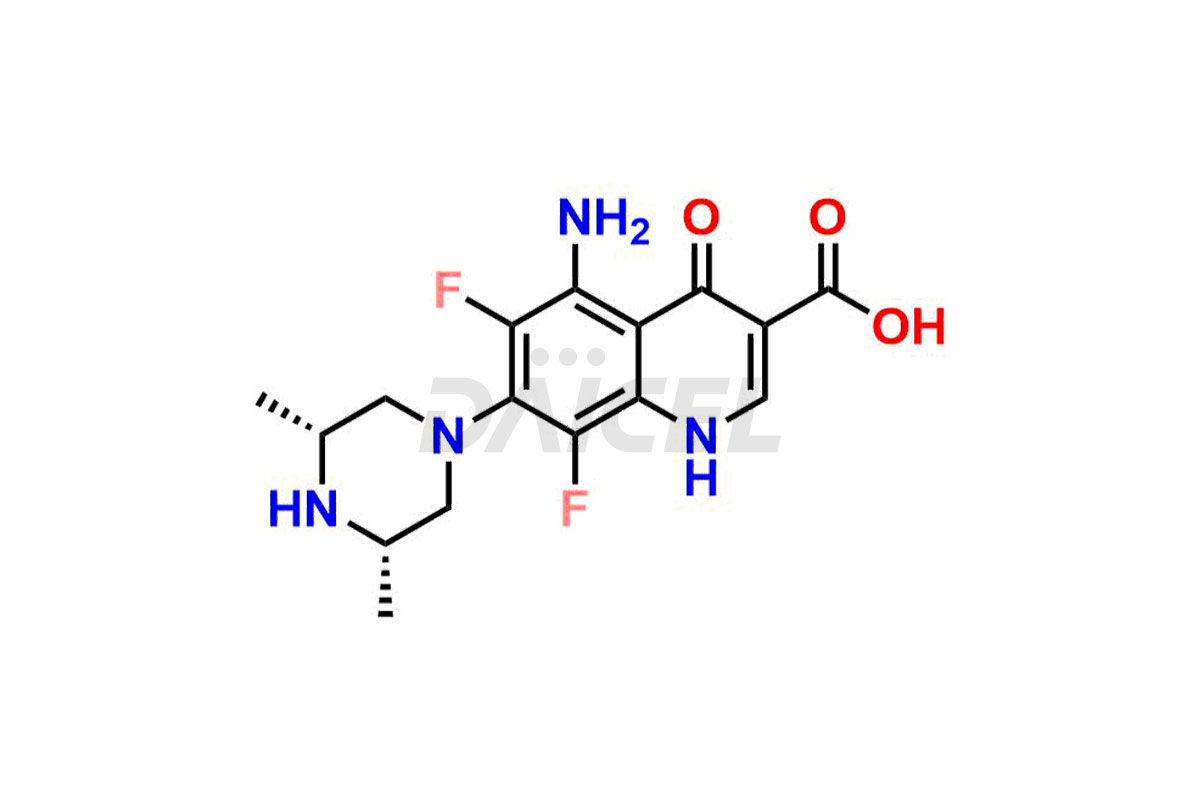
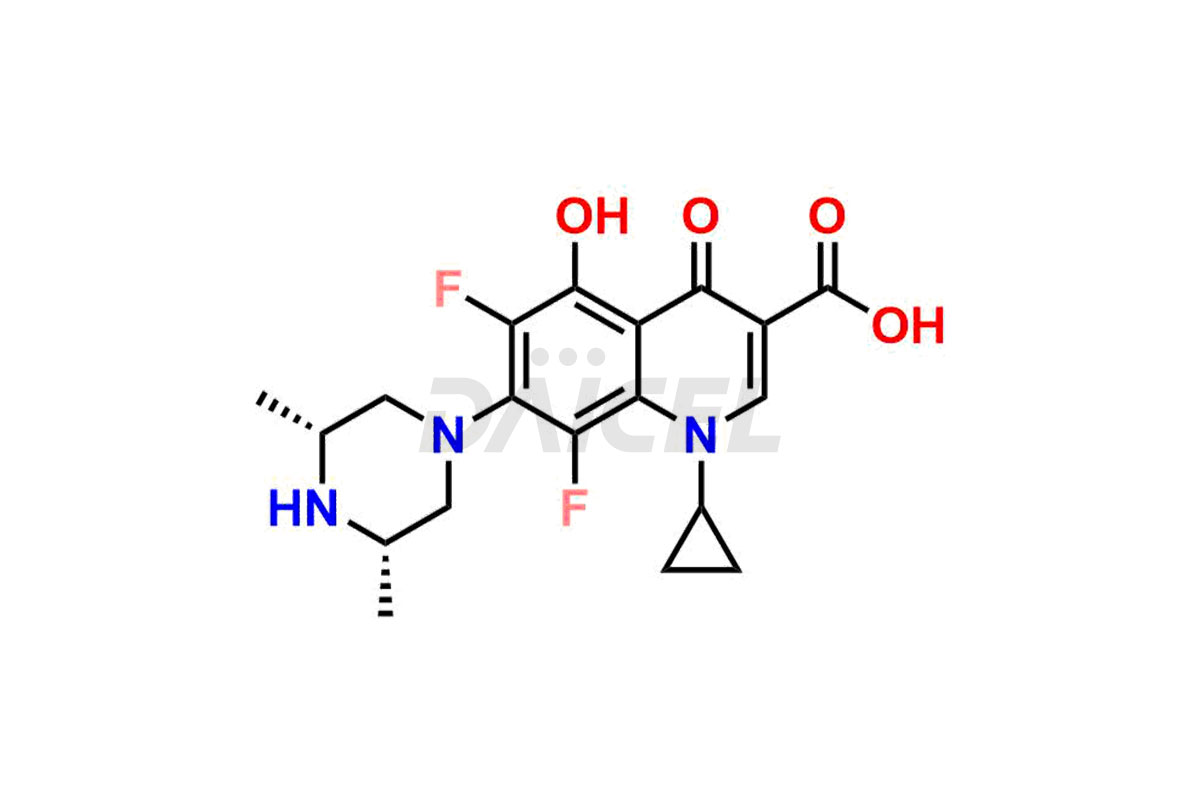
Sparfloxacin
General Information
Sparfloxacin Impurities and Sparfloxacin
Daicel Pharma offers a comprehensive range of exclusive Sparfloxacin impurities such as Sparfloxacin Benzyl amino Impurity, Sparfloxacin de-amino Impurity, Sparfloxacin De-cyclopropyl Impurity, Sparfloxacin Fluoro Impurity, Sparfloxacin Hydroxy Impurity, and Sparfloxacin Diamino Impurity. These impurities are critical in determining the active pharmaceutical ingredient Sparfloxacin’s quality, stability, and biological safety. Additionally, Daicel Pharma can synthesize Sparfloxacin impurities according to precise customer specifications while guaranteeing worldwide delivery.
Sparfloxacin [CAS: 110871-86-8] is an antibacterial that treats various bacterial infections, such as pneumonia.
Sparfloxacin: Use and Commercial Availability
Sparfloxacin is a fluoroquinolone antibiotic that treats bacterial infections. It treats various respiratory tract infections, including acute exacerbations of chronic bronchitis and community-acquired pneumonia caused by susceptible strains of microorganisms.
Sparfloxacin is available under Zagam, which effectively contains the active ingredient, Sparfloxacin.
Sparfloxacin Structure and Mechanism of Action 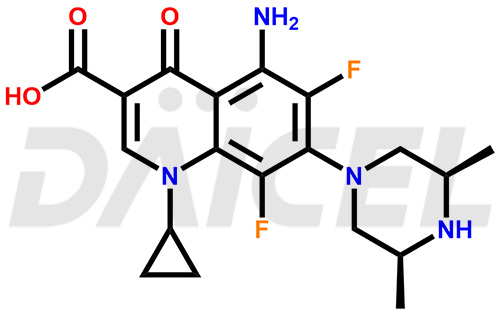
The chemical name of Sparfloxacin is rel-5-Amino-1-cyclopropyl-7-[(3R,5S)-3,5-dimethyl-1-piperazinyl]-6,8-difluoro-1,4-dihydro-4-oxo-3-quinolinecarboxylic acid. Its chemical formula is C19H22F2N4O3, and its molecular weight is approximately 392.4 g/mol.
Sparfloxacin functions by inhibiting the activity of bacterial enzymes, topoisomerase II (DNA-gyrase) and topoisomerase IV, involved in bacterial DNA replication, transcription, and repair.
Sparfloxacin Impurities and Synthesis
Impurities can arise during the synthesis1 and storage of Sparfloxacin, including related substances, degradation products, or residual solvents. These impurities need monitoring and control to ensure the medication’s safety, efficacy, and quality.
Daicel offers a Certificate of Analysis (CoA) from a cGMP-compliant analytical facility for Sparfloxacin impurity standards like Sparfloxacin Benzyl amino Impurity, Sparfloxacin de-amino Impurity, Sparfloxacin De-cyclopropyl Impurity, Sparfloxacin Fluoro Impurity, Sparfloxacin Hydroxy Impurity, and Sparfloxacin Diamino Impurity. The CoA includes complete characterization data, such as 1H NMR, 13C NMR, IR, MASS, and HPLC purity. We give additional characterization data, including 13C-DEPT, on request. Daicel Pharma specializes in synthesizing unknown impurities or degradation products of Sparfloxacin.
References
FAQ's
References
Frequently Asked Questions
How are Sparfloxacin impurities detected and quantified?
Methods such as High-Performance Liquid Chromatography (HPLC) can detect impurities in Sparfloxacin.
Can Sparfloxacin impurities affect patient safety?
Sparfloxacin impurities can impact patient safety. Depending on their nature and concentration, contaminants can cause adverse effects or reduce the efficacy of the medication.
Which solvents help in the analysis of Sparfloxacin impurities?
Acetonitrile, DMSO, or Methanol help achieve optimal solubility and separation of Sparfloxacin impurities.
What are the temperature conditions required to store Sparfloxacin impurities?
Sparfloxacin impurities are stored at a controlled room temperature between 2-8 ⁰C or as indicated on the Certificate of Analysis (CoA).
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.