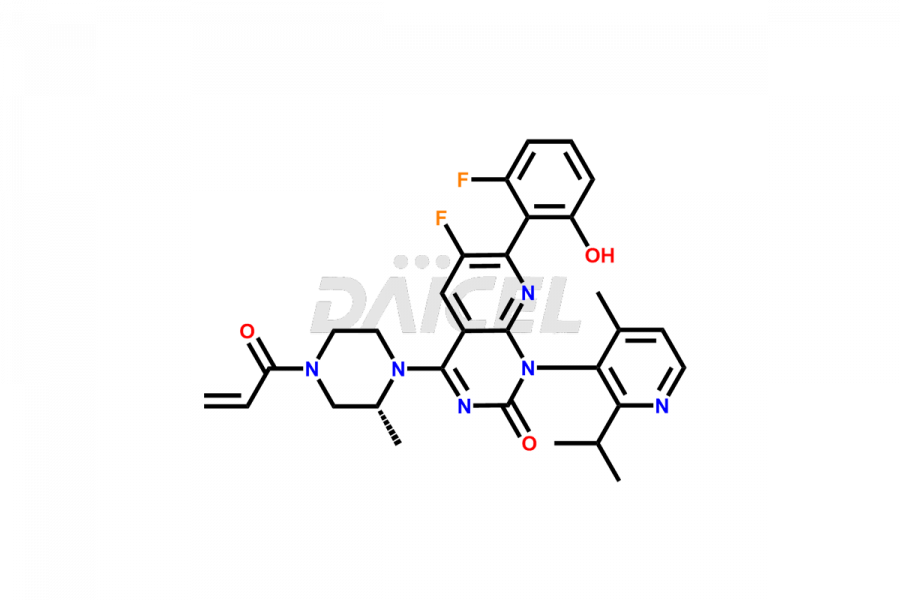
Sotorasib
General Information
Sotorasib Impurities and Sotorasib
Daicel Pharma synthesizes high-quality Sotorasib impurities like Sotorasib Isomer-3 and Sotorasib Isomer-4, which are crucial in the analysis of the quality, stability, and biological safety of the active pharmaceutical ingredient Sotorasib. Moreover, Daicel Pharma offers custom synthesis of Sotorasib impurities and delivers them globally.
Sotorasib [CAS: 2296729-00-3] is a pyridopyrimidine derivative that treats non-small cell lung cancer (NSCLC) in patients having KRAS(G12C) mutations. It inhibits growth in KRAS G12C tumor cell lines.
Sotorasib: Use and Commercial Availability
Sotorasib, or AMG 510, is a drug taken orally with the potential to treat certain types of cancer. It treats non-small cell lung cancer (NSCLC) in adults with advanced cancer and has a KRAS G12C mutation. It is available under the trade name Lumykras. Lumykras is a medicine for treating advanced NSCLC with KRAS G12C mutation in adults who have progressed after at least one prior line of systemic therapy. Amgen is the company that developed this drug.
Sotorasib Structure and Mechanism of Action 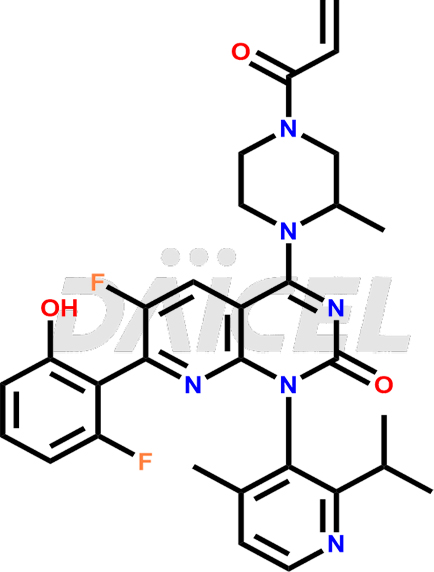
The chemical name of Sotorasib is (1R)-6-Fluoro-7-(2-fluoro-6-hydroxyphenyl)-1-[4-methyl-2-(1-methylethyl)-3-pyridinyl]-4-[(2S)-2-methyl-4-(1-oxo-2-propen-1-yl)-1-piperazinyl]pyrido[2,3-d]pyrimidin-2(1H)-one. Its chemical formula is C30H30F2N6O3, and its molecular weight is approximately 560.6 g/mol.
Sotorasib inhibits KRASG12C, a mutant-oncogenic, tumor-restricted form of the RAS GTPase, KRAS. It covalently binds with the cysteine of KRASG12C blocks KRAS signaling, slows cell growth, and allows apoptosis only in KRAS G12C tumor cell lines.
Sotorasib Impurities and Synthesis
Sotorasib is a promising targeted therapy for non-small cell lung cancer with a specific mutation, but it may produce impurities during synthesis1. These impurities may affect the drug’s purity, stability, and efficacy. Therefore, it is crucial to identify the potential impurities, understand their formation, and develop strategies to control and monitor them to ensure the drug’s quality and safety.
Daicel provides a Certificate of Analysis (CoA) for Sotorasib impurity standards, Sotorasib Isomer-3, and Sotorasib Isomer-4. The CoA is issued from a cGMP-compliant analytical facility and contains complete characterization data, such as 1H NMR, 13C NMR, IR, MASS, and HPLC purity2. Additional characterization data, such as 13C-DEPT and CHN, can be provided upon request. Daicel can also prepare any unknown Sotorasib impurity or degradation product. We give a complete characterization report on delivery.
References
FAQ's
References
- Lanman, Brian Alan; Chen, Jian; Reed, Anthony B.; Cee, Victor J.; Liu, Longbin; Kopecky, David John; Lopez, Patricia; Wurz, Ryan Paul; Nguyen, Thomas T.; Booker, Shon; et al, KRAS G12C inhibitors and methods of using the same, Amgen Inc., United States, US10519146B2, December 31, 2019
- Wong, Philip; Akrami, Anna; Houk, Brett; Vuu, Irene; James, Christopher A., Validation of an LC-MS/MS method for the determination of sotorasib, a KRASG12C inhibitor, in human plasma, Bioanalysis, Volume: 14, Issue: 19, Pages: 1281-1292, 2022
Frequently Asked Questions
How are Sotorasib impurities formed?
Impurities form during the synthesis of Sotorasib or storage. They can also occur due to chemical reactions with other substances.
What are the most common Sotorasib impurities?
The most common impurities found in Sotorasib are related compounds, degradation products, and residual solvents.
Which solvent helps in the analysis of Sotorasib impurities?
DMSO is the solvent used in analyzing many impurities in Sotorasib.
What are the temperature conditions required to store Sotorasib impurities?
Sotorasib impurities are stored at a controlled room temperature between 2-8 ⁰C or as indicated on the Certificate of Analysis (CoA).
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.