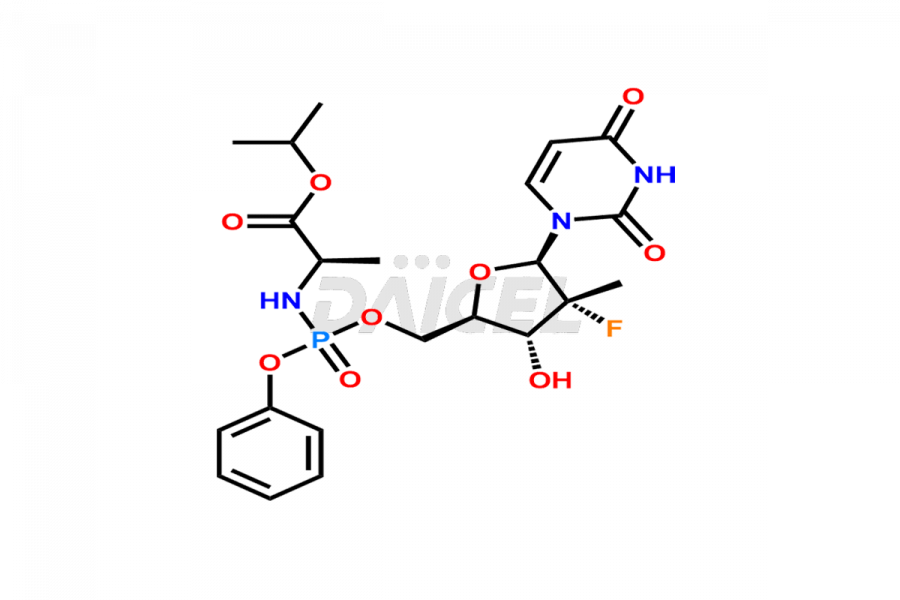
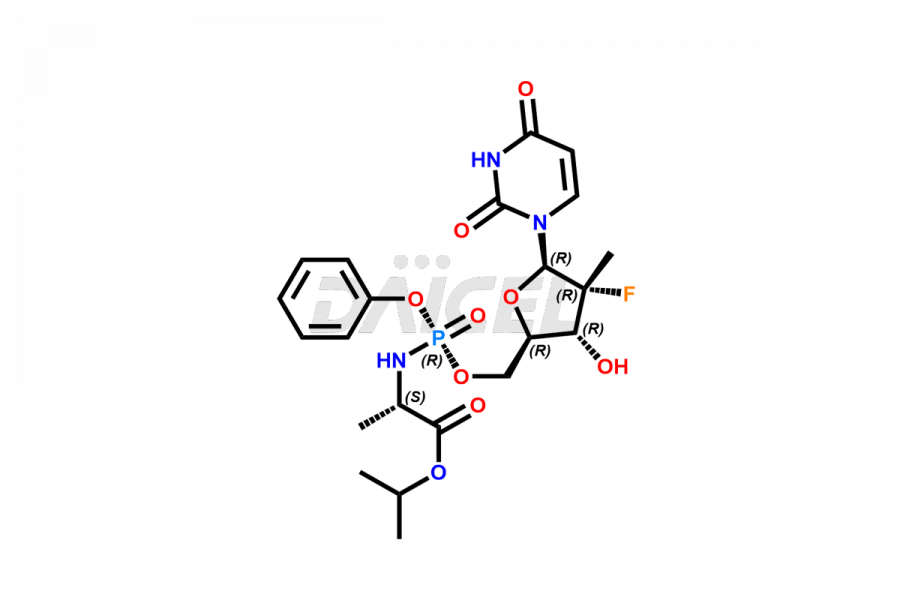
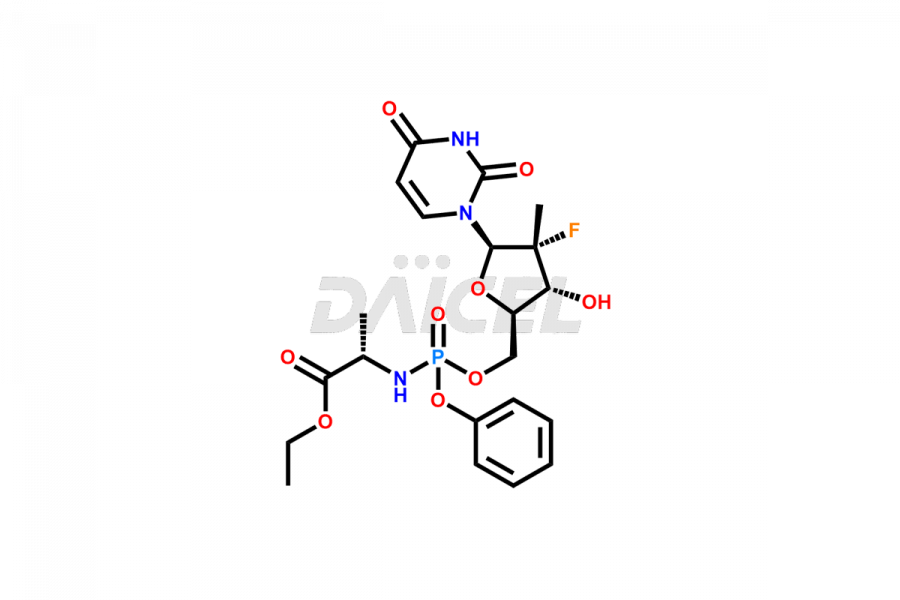
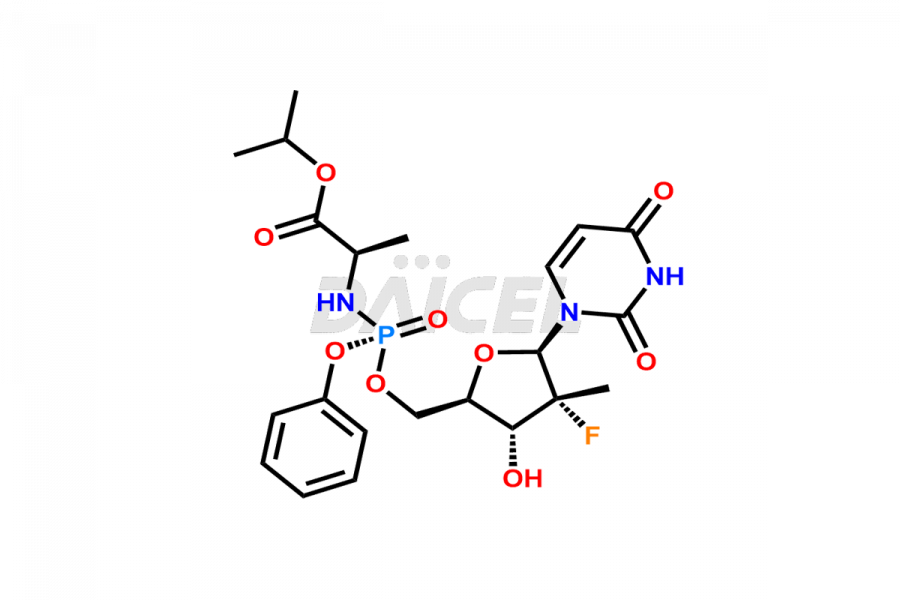
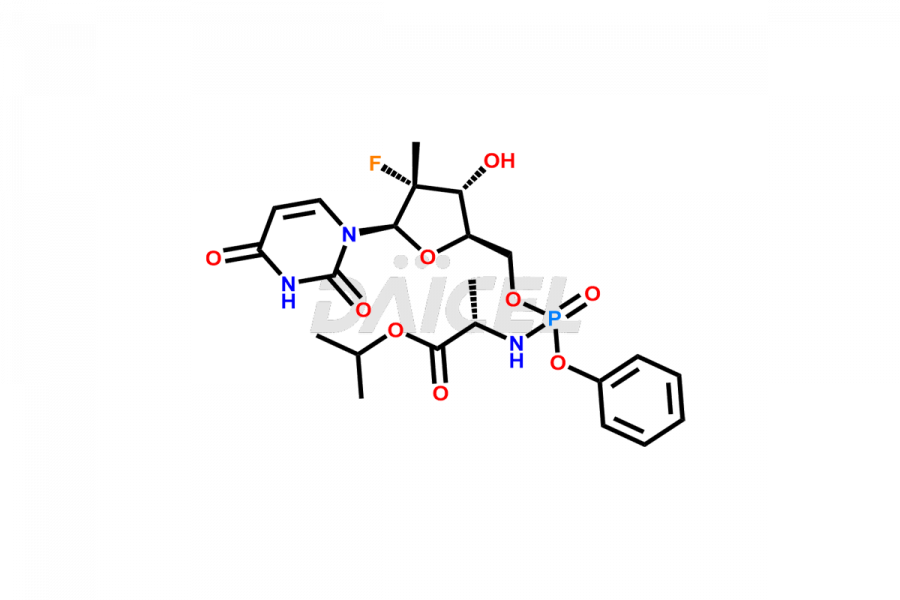
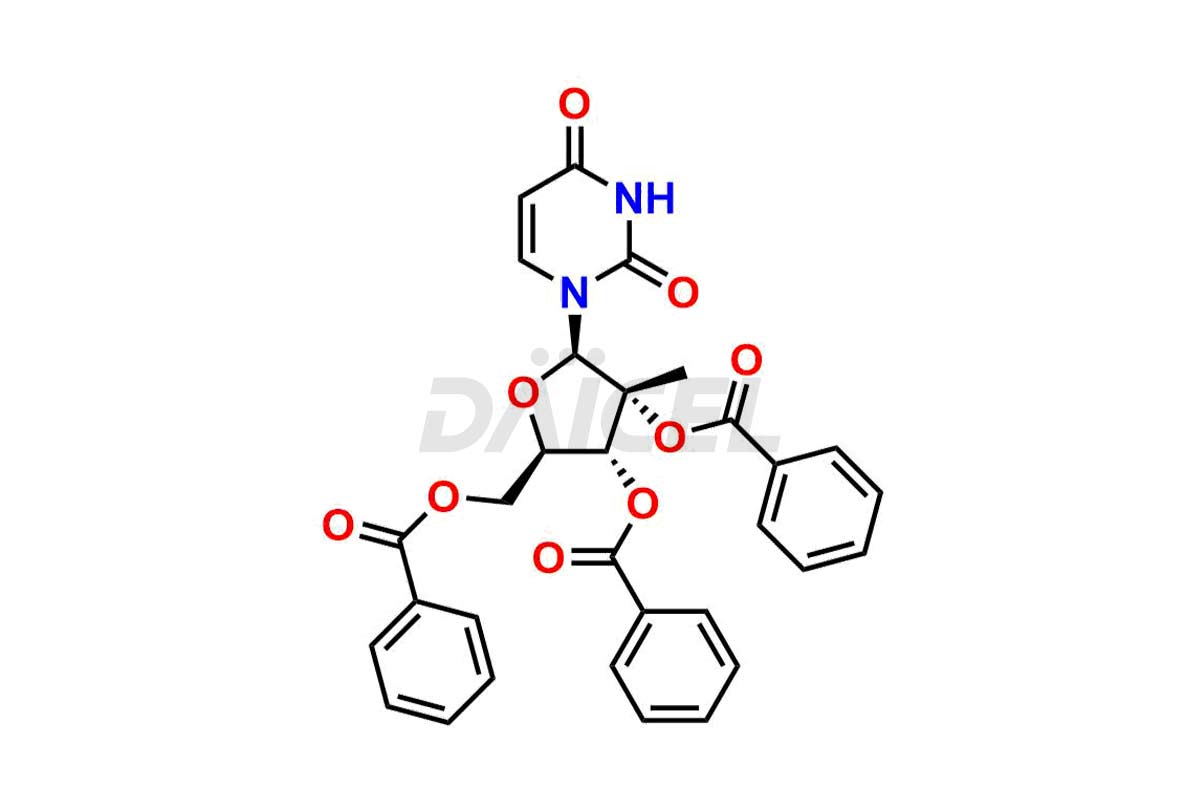
Sofosbuvir
General Information
Sofosbuvir Impurities and Sofosbuvir
Daicel Pharma offers a comprehensive range of exclusive Sofosbuvir impurities such as D-Alanine Sofosbuvir, Sofosbuvir (R)-Phosphate, Sofosbuvir D-alaninate R, S Isomer, Sofosbuvir Isomer 3, Sofosbuvir Impurity, and more. These impurities are critical in determining the active pharmaceutical ingredient Sofosbuvir’s quality, stability, and biological safety. Additionally, Daicel Pharma can synthesize Sofosbuvir impurities according to precise customer specifications while guaranteeing worldwide delivery.
Sofosbuvir [CAS: 1190307-88-0] is an orally administered nucleoside analog prescribed with other antiviral medications It treats chronic Hepatitis C Virus (HCV) infection in patients with HCV genotypes 1,2, 3,4, 5, 6. It is also effective in treating patients co-infected with HCV and HIV.
Sofosbuvir: Use and Commercial Availability
Sofosbuvir is a widely used antiviral medication available for treating chronic hepatitis C virus (HCV) infections. It combines with other antiviral drugs as a comprehensive therapy regimen. Sofosbuvir is administered orally, making it convenient for patients. It has been proven highly effective in achieving sustained virologic response, improving patient outcomes.
Sofosbuvir is available under Sovaldi, which contains the active ingredient, Sofosbuvir.
Sofosbuvir Structure and Mechanism of Action 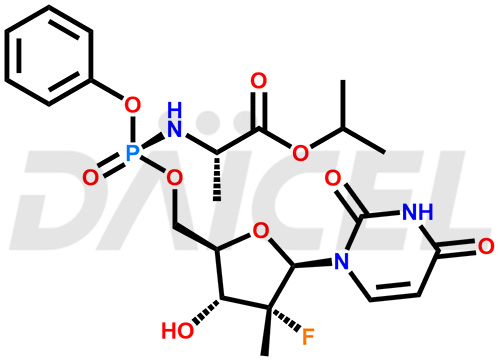
The chemical name of Sofosbuvir is N-[[P(S),2′R]-2′-deoxy-2′-fluoro-2′-methyl-P-phenyl-5′-uridylyl]- L-Alanine 1-methylethyl ester. Its chemical formula is C22H29FN3O9P, and its molecular weight is approximately 529.5 g/mol.
Sofosbuvir prevents the HCV NS5B RNA-dependent RNA polymerase that causes viral replication.
Sofosbuvir Impurities and Synthesis
During the synthesis1 and storage of Sofosbuvir, impurities can arise, including related substances, degradation products, and residual solvents. It is essential to carefully monitor and control these impurities to ensure the medication’s safety, efficacy, and overall quality.
Daicel Pharma offers a comprehensive Certificate of Analysis (CoA) for Sofosbuvir impurity standards such as D-Alanine Sofosbuvir, Sofosbuvir (R)-Phosphate, Sofosbuvir D-alaninate R, S Isomer, Sofosbuvir Isomer 3, Sofosbuvir Impurity, and more. These impurities form in compliance with current Good Manufacturing Practices (cGMP). The CoA includes detailed characterization data, such as 1H NMR, 13C NMR, IR, MASS, and HPLC purity2, providing a thorough understanding of the impurity profile. Upon request, Daicel can also provide 13C-DEPT data for further characterization.
Furthermore, Daicel Pharma possesses the technical expertise to synthesize any unknown impurities or degradation products of Sofosbuvir. We also offer labeled compounds. For bioanalytical research and Bioavailability/Bioequivalence (BA/BE) studies, Daicel Pharma supplies Sofosbuvir-D6, a highly pure deuterated-labeled standard of Sofosbuvir.
References
FAQ's
References
Frequently Asked Questions
How are Sofosbuvir impurities detected and quantified?
Analytical Methods such as Reverse Phase-High Performance Liquid Chromatography (RP-HPLC) can detect impurities in Sofosbuvir.
Can Sofosbuvir impurities affect patient safety?
Impurities in Sofosbuvir can impact patient safety. Depending on their nature and concentration, contaminants can cause adverse effects or reduce the efficacy of the medication.
Which solvents help in the analysis of Sofosbuvir impurities?
Acetonitrile helps achieve optimal solubility and separation of Sofosbuvir impurities.
What are the temperature conditions required to store Sofosbuvir impurities?
Sofosbuvir impurities are stored at a controlled room temperature between 2-8 °C or as indicated on the Certificate of Analysis (CoA).
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.